Addition / Reduction
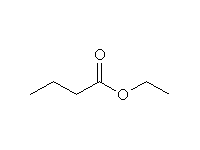
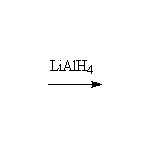
 +
+
Other reagents: H2 / Pt, or similar catalyst; NaBH4 is a weaker reducing agent and reacts slower with esters than with ketones so that selective reductions can be done.
McMurry 21.5, Fessenden 15.5C, Schmid 15.17
To other syntheses of alcohols

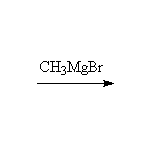
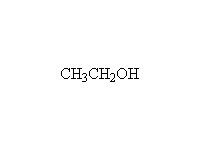
 +
+

Note that two moles of Grignard reagent are required to do this reaction. The product from addition of one mole of Grignard is a ketone which also reacts with Grignard. If two moles of Grignard are not present, incomplete reaction yields a mixture of products and starting materials.
McMurry 21.5, Fessenden 7.3D, 15.5C, Schmid 16.13
To other syntheses of alcohols
Addition / Elimination

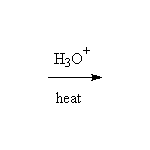
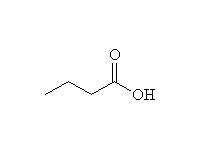 +
+

This reaction is known as hydrolysis.
Base-catalyzed hydrolysis, i.e. with H2O, OH-, is known as saponification
McMurry 21.6, Fessenden 15.5C, Schmid 15.11, 15.12
To other syntheses of carboxylic acids

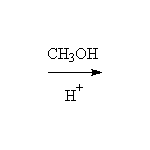
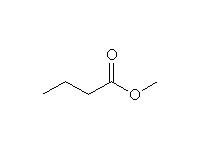 +
+

This reaction is known as interesterification or trans-esterification.
Other reagents: any other alcohols
McMurry 21.5, Fessenden 15.5C, Schmid 16.9

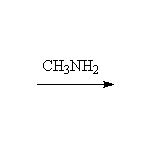
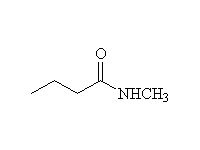 +
+

McMurry 21.5, Fessenden 15.5C, Schmid 16.9
To other syntheses of amides
To synthesis of esters
To the main graphical reactions menu.
Last update April 1999