B2H6 is the actual molecular formula for a reagent whose simplest formula is BH3. The boron would have only 6 electrons in BH3; its attempt to get 8 electrons results in its sharing hydrogens and their electrons in a bridged structure shown below.
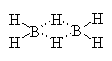
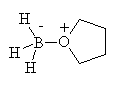
You will often find this reagent referred to as just BH3, since it is commonly sold in solution as a complex with a base such as methylamine or in solution of a moderately high boiling ether such as THF(tetrahydrofuran) with which it also forms a complex, shown above.
B2H6 does not behave as a source of hydride; instead it adds to carbon-carbon double bonds in a concerted manner to place the boron (somewhat positive) on the less substituted carbon and the hydrogen (somewhat negative) on the more substituted carbon (better able to support a sl ight positive charge as the electrons are being reshuffled in the transition state). This addition results in an addition to (a reduction of) the double bond which puts the heteroatom on the less substituted carbon.

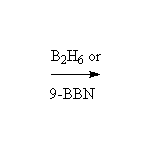
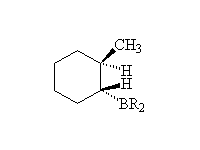
Since boron is less electronegative than carbon, the B is somewhat positive, and can only be replaced by other slightly positive things. Useful things like halides and hydroxides are negative and won't work; instead sources are needed which can produce positive halogen or oxygen - oxidizing agents! Reaction with bromine or chlorine (Br2, Cl2) produces the less substituted halide from the alkene, reaction with hydrogen peroxide (HOOH) the alcohol and chloramine (NH2Cl) the amine. See the essays on halogens and peroxides as oxidizing agents.
Because the reaction of the B-H bond with the C=C bond is concerted, it is also stereospecific. Moreover, the subsequent reaction of the oxidizing agent retains that stereochemistry. Thus, B2H6 provides a method for producing not only the less substituted alcohol, etc., but only one diastereomer thereof. This control of both the regiochemistry and stereochemistry is important in the synthesis of natural products such as pheromones and antibiotics.
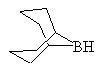
To improve the selectivity of the boron for the less substituted carbon, a bulky group can be added; the most common reagent of this type is 9-borabicyclo[3.3.1]nonane, 9-BBN, below. B2H6 can also be used to reduce carbonyl groups, but it is less convenient to use than NaBH4.
Precautions for B2H6: Similar to NaBH4 and LiAlH4, with extra care since B2H6 is a gas. The ether solutions are of course flammable.
Dihydrogen
Since reduction is defined as addition of hydrogen, dihydrogen (H2) would seem to be the ideal reducing agent. However, the strength of the H-H bond and the lack of polarizability of the molecule makes it extremely unreactive. Fortunately some precious metals - platinum, palladium, nickel - "react" with dihydrogen in a rather unusual way. The metals dissolve dihydrogen and partially bond to it, effectively breaking the H-H bond; platinum will dissolve more than a mole of hydrogen and swells visibly in the process - it is like a sponge. Thus these metals serve as catalysts for reactions of dihydrogen. Typically dihydrogen adds to multiple bonds - alkenes, alkynes, carbonyl compounds - in the presence of these catalysts. Since the hydrogenation reaction takes place on the surface, it is stereospecific syn.
By careful control of the reaction conditions and the exact nature of the catalyst, it is possible to reduce one kind of multiple bond without some others that are present reacting. An alkyne can be reduced to a Z (cis) alkene by "poisoning" the Pt catalyst so that the addition stops at the alkene; reagents that have been used are sulfur compounds such as barium sulfate, organic amines such as quinoline. A weakened palladium catalyst called Lindlar's catalyst (Pd with CaCO3 and Pb(OAc)2 is very popular too. With a weaker catalyst or much milder conditions, it is possible to reduce an alkene without reducing a carbonyl in the same molecule, even if they are conjugated.
Metals and Organometallic Reagents
The Grignard Reagent
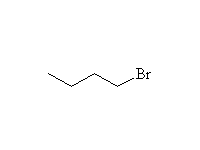
Reaction of an alkyl halide with magnesium metal in diethyl ether results in the formation of an organometallic compound with the magnesium replacing the halide; the second valence of the magnesium (II) is satisfied by the halide removed from the carbon.
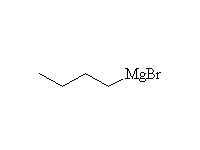
The carbon has been changed from positive to negative and has been reduced; magnesium, the reducing agent, has been oxidized. This reaction changes the normal polarity of carbon in organic compounds, and that negative carbon produced is very reactive toward positive sites. The most useful reaction of the negative carbon of the Grignard is addition to carbonyl groups. It reduces aldehydes, ketones, esters, carboxylic acid chlorides to alcohols and nitriles to amines. It is unselective, adding twice to esters and carboxylic acid chlorides. Thus aldehydes give secondary alcohols, and ketones, esters, and carboxylic acid chlorides give tertiary alcohols. Nitriles give primary amines with a tertiary carbon (see amine nomenclature).
Precautions: The negative carbon of the Grignard reagent will react with almost anything that is positive; for example: the OH's of water, alcohols, carboxylic or other acids, the NH's of amines or amides, the N of nitro groups. It is essential that glassware and reagents be completely dry; the complex mechanism of Grignard formation can be completely halted by traces of water. There are also severe restrictions on the functional groups that can be in the molecule the Grignard is made from or reacting with (no polar double bonds or slightly acidic hydrogens). In addition, ether (diethyl ether or tetrahydrofuran, THF) is essential to stabilizing the Grignard reagent; if the solution becomes too concentrated the Grignard will react with the somewhat positive carbon of the starting halide as a nucleophile.
 +
+
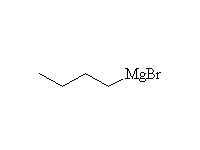
 (no ether)
(no ether)
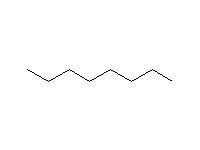
Aryl (aromatic) halides may also be converted into Grignard reagents; bromobenzene generates phenylmagnesium bromide, which reacts just like butylmagnesium bromide. The only restriction is that chlorobenzene is too unreactive and can only be converted to the corresponding Grignard reagent at higher temperatures, e.g. using THF as a solvent. Alkyl Grignards are most easily prepared from primary halides, followed by secondary; tertiarys are difficult; the negative carbon is responsible for these differences (compare the order of stability of positive carbon in carbocations). Halide reactivity toward magnesium is in the order I>Br>Cl.
Dialkylcopper Lithium (Lithium Dialkylcuprate) Reagents
The dialkyl copper lithium reagents are often made from Grignard reagents.
R2CuLi reagents are less reactive and more selective than Grignard reagents. They react with aldehydes and ketones only slowly but with carboxylic acid chlorides very quickly. As a result they can be used to reduce carboxylic acid chlorides to ketones without further reduction to the tertiary alcohol. Since they are often made from the Grignard reagent, the same precautions and restrictions on structure apply. Note that their selectivity is like that of LiAl(OtBu)3H, discussed above, but of course the variable R group gives them more versatility.
Sodium, Alkyl Sodium and Alkyl Lithium
Treatment of alkyl halides with sodium results in a rapid oxidation of the metal, but the negative carbon of the corresponding RNa reacts with the positive carbon of the remaining RX to give RR (like a Grignard does without the ether); this is called coupling, or sometimes the Wurtz reaction. RLi's are more manageable and is used as nucleophilic reagents for addition to carbonyl groups. More often, they are just used as strong bases, e.g. to convert an alkyne to its conjugate base.
Treatment of compounds with even slightly acidic protons with sodium metal will result in the same reaction as occurs with the acidic hydrogen of water, namely reduction of that somewhat positive hydrogen to H2 and oxidation of the sodium to Na+. Useful examples include: alcohol to alkoxide (base and nucleophile), and alkyne to alkynide (nucleophile).
If you look at the reducing agents above, you will note that they are all basic. But there is a family of reducing agents that are acidic - a moderately reactive metal with hydrochloric acid. The Clemmensen reduction uses a liquid amalgam (metal solution) of zinc and mercury with HCl to reduce ketones to hydrocarbons. Tin (Sn) or iron (Fe) with HCl can be used to reduce nitro groups to amino groups; this reduction is especially useful for making anilines since the nitrobenzenes are easy to make.
Last revised October 2001. Copyright 1998 Linda M. Sweeting
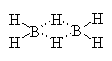
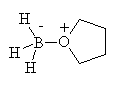

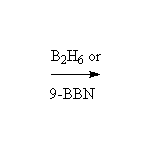



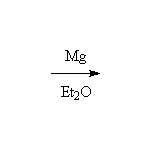
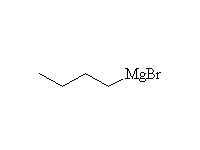
 +
+
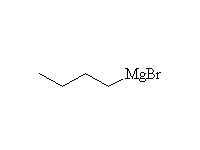
 (no ether)
(no ether)