From Alkyl Halides - Nucleophilic Substitution
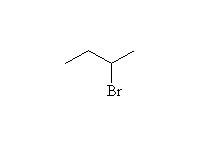
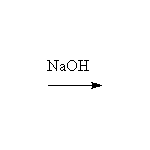

Mechanism! SN2 reaction.
Will be SN1 for tertiary halides (carbocations, rearrangements)
Competition also from E2 elimination in non-aqueous solvents
McMurry 7.1, 10.7, 11.9, 17.7, Fessenden 5.4, 5.8, 5.9; Schmid 12.1, 12.2
From Alkenes: Addition
 + H2O
+ H2O
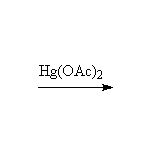
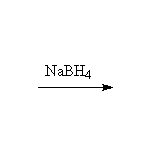
Oxidation, followed by reduction, giving the more substituted alcohol.
Other reagents: acid and water, but these simple addition conditions give rearrangements if a more stable carbocation is possible.
McMurry 7.4, Fessenden 10.8; Schmid 7.14, 8.10

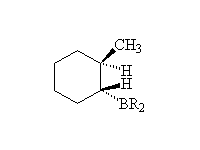

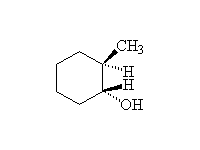
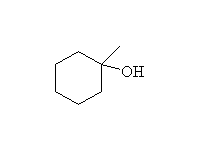
Reduction followed by oxidation, giving the less substituted alcohol.
McMurry 7.4, Fessenden 10.9; Schmid 8.1 - 8.4

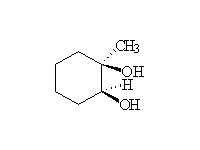
Other reagents: KMnO4 with no heat. Heating an alkene with basic KMnO4 results in cleavage of the double bond to form ketones and carboxylic acids.
McMurry 7.8, Fessenden 10.13A & B; Schmid 8.5

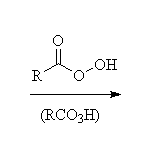
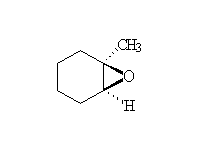

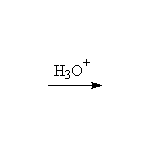
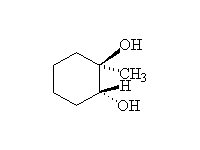
Other reagents: aqueous sodium hydroxide for epoxide opening. Both reactions are stereospecific and usually SN2 - the base-catalyzed reaction occurs predominantly at the less substituted carbon, but the acid-catalyzed addition occurs predominantly at the more substituted carbon (if the starting material is a single enantiomer, the products of these two methods of opening the ring are the enantiomers).
McMurry 7.4, 17.4, Fessenden 8.2B & C, 10.13A, Schmid 8.6, 13.12

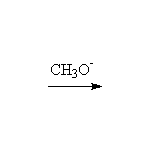
Stereospecific! Base-catalyzed ring opening is by SN2, at the less substituted carbon. Note that other nucleophiles, such as thiols, thiolates, cyanide, also will open the ring.
McMurry 7.4, Fessenden 8.4A, Schmid 13.12

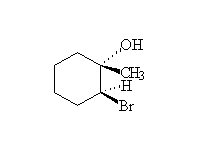
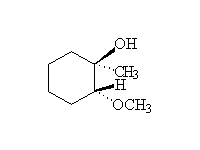
Mechanism!
McMurry 7.4, Fessenden 10.10; Schmid 8.9
From Carbonyl Compounds: Addition
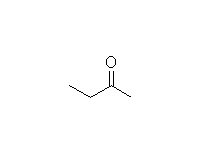
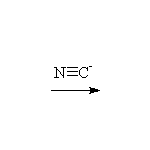
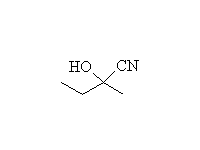
Mechanism!
McMurry 6.8, 6.9, 7.2, 9.17, Fessenden 13.4, Schmid 14.9

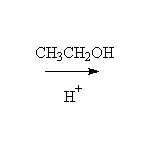
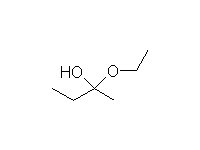
A hemiketal (hemiacetal): half alcohol and half ether; unstable to conversion to ketal or ketone, depending on conditions.
Mechanism! Reversible
Fessenden 13.4B Schmid 14.11
From Carbonyl Compounds: Addition with Reduction

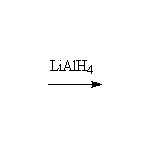

Other reagents: NaBH4, H2with Pt
Fessenden Schmid 11.7
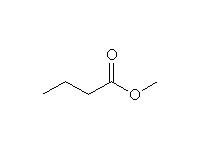
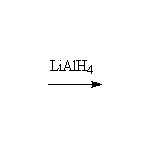
 + CH3OH
+ CH3OHOther reagents: NaBH4, H2with Pt
Other substrates: This reaction also works well with acid chlorides. However, if you use a carboxylic acid as reagent, the acidic hydrogen reacts first, converting the substrate to an anion and making it les reactive. Only LiAlH4 can do this reduction.
Fessenden 15.5, 15.3, 14.7; Schmid 15.17

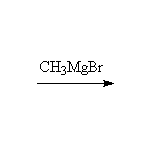
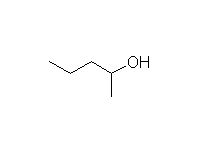

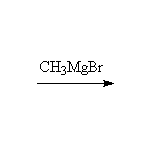
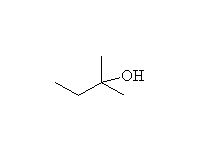
Fessenden 7.3D, 13.4D, Schmid 11.7
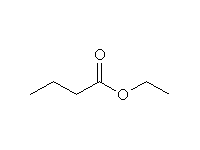
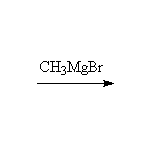
 +
+
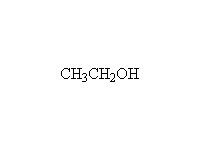
Note that two moles of Grignard reagent are required to do this reaction. The product from addition of one mole of Grignard is a ketone which also reacts with Grignard. If two moles of Grignard are not present, incomplete reaction yields a mixture of products and starting materials.
McMurry 10.8, Fessenden 15.5; Schmid 16.13
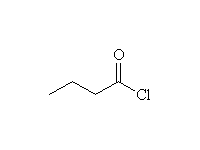
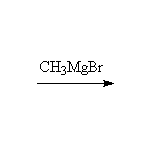

Note that two moles of Grignard reagent are required to do this reaction. The product from addition of one mole of Grignard is a ketone which also reacts with Grignard. If two moles of Grignard are not present, incomplete reaction yields a mixture of products and starting materials.
Fessenden 15.3; Schmid 16.13
To: Reactions of carboxylic acid chlorides
Back to the main graphical reactions menu