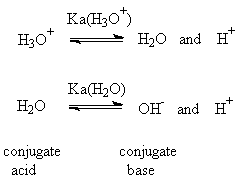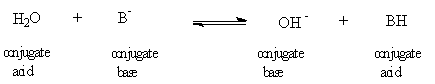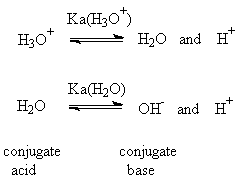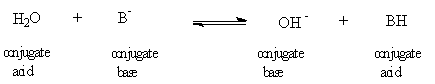All bases will react with both protic (Bronsted) acids or Lewis acids, as you will see in the wide variety of reactions of organic chemistry. There are two main distinguishing classes of bases: aqueous vs non-aqueous and "hard" vs "soft". IN water, the base strength of the solution is reduced or "levelled" (see the essay on acids for more on "levelling") to that of hydroxide, but in non-aqueous solutions stronger bases are commonly observed; even hydroxide itelf is a stronger base when dissolved in a solvent other than water. Although the behavior of bases depends on the solvent, it is convenient to classify as "hard" those bases which prefer to react with oxygen, nitrogen and other electronegative, non-polarizable atoms and to classify as "soft" those that react with less electronegative, more polarizable atoms such as the carbons in organic compounds. Thus soft bases are also called nucleophiles. See the discussion of halides below for more about the effect of solvent on the strength of nucleophiles.
Aqueous Bases and Nucleophiles
Water and Hydroxide, H2O and OH-
Most students' experience in chemistry before organic is confined to aqueous solutions, where water and hydroxide reign supreme. Any other bases dissolved in water react with that slightly acidic proton of water and convert it to hydroxide, the strongest base you can have in water (see above for more on this). Both water and hydroxide are pretty good nucleophiles and bases. For example, hydroxide is able to substitute alkyl halides if they are not too hindered; however, aqueous hydroxide is usually not basic enough to convert an alkyl halide into an alkene (a dehydrohalogenation). Hydroxide is actually a stronger base in other solvents (if you can get it dissolved); we believe that the hydrogen-bonding network of water reduces hydroxide's basicity. As a general-purpose base, hydroxide is used to convert moderately acidic compounds (e.g. carboxylic acids and ketones (a-H's)) to the conjugate bases.
Alcohols
As bases, alkanols (ROH) are slightly stronger than water, because of the electron-donating effect of the alkyl groups (phenols are less basic and more acidic because of the electron-withdrawing effect of the benzene ring). As solvents, alcohols are not as good at hydrogen bonding as water (only one H per molecule) and thus they do not have as big a leveling effect on the basicity of solutes. KOH dissolves fairly well in ethanol and is successful at carrying out dehydrohalogenations on all but the least reactive alkyl halides. A better choice for dehydrohalogenations is the alkoxide salt of the alcohol solvent - it is more basic still and can be selected for steric hindrance to maximize the yield of the less substituted alkene if that is desired. The steric hindrance combined with weak basicity makes alcohols a poor choice for an SN2 reaction, but they are good nucleophiles for the less hindered and more positive carbonyl group, converting aldehydes into acetals and acids into esters when the carbonyl is protonated; no acid catalysis is needed with the highly reactive acid chlorides.
Ammonia, NH3, and Amines
In aqueous solution, ammonia and amines are partially converted into their conjugate acids and hydroxide (neutral example in the equation with B above), but even in aqueous solution, they are much better nucleophiles than the components of water. For example, a primary amide can be made by pouring an acyl halide into concentrated aqueous ammonia (28% - ammonia itself is a gas with a boiling point of -33 C). Substituted amides, though, are made by dissolving the correct amine in an inert organic solvent. (Amines with more than about 5 carbons are not soluble in water and thus must be dissolved in inert organic solvents). It is tempting to use these bases to make amines by an SN2 reaction on an alkyl halide. However, the amine that is formed is at least as basic and nucleophilic as the starting ammonia or amine and reacts with other molecules of halide to put a second - or more - alkyl groups on the nitrogen. This complication is not observed when converting halides to alcohols as the oxygen is not basic enough or nucleophilic enough to react (recall that the best reagent for converting halide to alcohol is not water but hydroxide, and alkoxide is needed to convert halide to ether). Pure primary amines can be made by the subterfuge of the Gabriel synthesis.
Ammonia and amines will also act as nucleophiles on carbonyl groups. A rapid exothermic reaction with an acid chloride converts an acid chloride into an amide. Slower reactions convert esters into amides and ketones and aldehydes into imines (the latter two reactions need to be done under anhydrous conditions to obtain any of the nitrogen-containing products). Amines which have low basicity such as 2,4-dinitrohydrazine will react very nicely with aldehydes and ketones in acidic solution (the acid protonates the carbonyl and thus catalyzes the reaction) to make imine-like compounds, in this case a yellow-to-red hydrazide.
CN-
Cyanide is an excellent nucleophile, and, although a fairly strong base, can be used as a nucleophile in aqueous solution. The SN2 replacement of a halide by cyanide is important for two reasons: 1) it adds a carbon to the chain, and 2) the cyanide can be converted readily to other functional groups by reduction (to amine) or hydrolysis (to amide and carboxylic acid). Cyanide is used in a classic synthesis of carbohydrates, elongating the chain of a starting sugar's by adding to the aldehyde group. It is important to ensure that the solution is basic when you are working with cyanide, because its conjugate acid, HCN, is a volatile (bp 25 C), extremely poisonous gas with a pleasant almond aroma. Cyanide in solution is deadly too, but you would have to swallow it. Great care is needed to work with cyanide.
Halide, especially I- and Br-
Because the hydrohalic acids are strong acids, the halide ions themselves are weak bases. The acidity order is HI > HBr > HCl and thus the basicity order is Cl->Br->I-. It is somewhat of a shock to find, then, that the order of nucleophilicity (ability to be a nucleophile) is I->Br->Cl-! Why? Experiments in the gas phase and in non-protic solvents like acetone and DMSO have the opposite order of reactivity, showing clearly that the water usually used as a solvent is the reason. Cl- is a relatively small ion and is coated with hydrogen-bonded water molecules which lower its electrostatic potential energy. The water molecules must be removed for the chloride to react as a nucleophile, and that process takes energy to break the hydrogen bonds. As the size of the ion increases, the water coat gets slimmer and the ion becomes more reactive. So far as we can tell, iodide is essentially naked in aqueous solutions, with no hydrogen-bonded water at all. There is an alternative explanation for the order of nucleophilicity of the halides, namely that iodide is "softer", more polarizable, and thus can stretch out and bond relatively far from the carbon it is reacting with. Chloride, being "harder" has to get very close to begin bonding. Although the concept of "hard" and "soft" has been successfully used for other reactions, it is clearly not the correct explanation in this case, since the most basic - chloride - is the most reactive if there is no water present.
Halide ions are not strong enough bases to replace hydroxide under normal conditions. In concentrated acid, they will displace OH (as water, not as hydroxide) either by an SN2 or an SN1 reaction (especially with the help of ZnCl2, the Lucas Reagent). To replace OH without strong acid requires either SOCl2 or PCl3. These reagents work for both the formation of alkyl halides and acyl halides.
Strong, Non-aqueous Bases
Probably the best way to get an inkling of the strength of a non-aqueous base is to look at the acidity of the conjugate acid. The conjugate acid is given for the four strong bases below, three of which would never be thought of as potential acids.
Alkoxides, RO-
Alkoxides (whose conjugate acids are alcohols) are somewhat stronger bases than hydroxide. Although they are good nucleophiles, you need to be careful in their use as their base strength tends to dominate their reactivity. For example, when brought into contact with alkyl halides, they usually cause an elimination reaction (base reacting with a hydrogen which can be used to convert it to its conjugate acid). It is only possible to use them as nucleophiles with primary halides (the Williamson synthesis of ethers); in this situation the nucleophilic substitution is fast enough to beat out the elimination reaction. Hindered alkoxides can be used to favor the less substituted of two possible alkenes.
Alkoxides can also be used to make enolates from aldehydes and ketones that are not very acidic. Their acid-base properties are similar, though, so the reaction is far from quantitative, and both enolates will be formed from an unsymmetrical ketone. If the enolate is much more stable (a weaker base) than the alkoxide, e.g. if stabilized by two carbonyls, the equilibrium is so biased as to form the enolate essentially quantitatively. Esters can also be converted to enolates; to avoid changing the identity of the alcohol part of the ester, it is essential to use the conjugate base of that alcohol to make the enolate, e.g. for a Claisen condensation (this reaction tells you that alkoxides add to carbonyls too, but the reaction is reversible and the product less stable than the original reagents)
Enolates
The conjugate bases of aldehydes and ketones are used as nucleophiles to substitute alkyl halides and add to carbonyl groups. Unsymmetrical ketones present an interesting problem - which enolate will be formed? It turns out that the less substituted enolate is formed faster but the more substituted enolate is more stable. So with alkoxide as the base, the more substituted enolate is formed and reacts, but with the powerful base LDA (see amide ions below), the conversion is essentially irreversible and the more rapidly formed, less substituted enolate is formed exclusively. The reaction of enolates with halides requires this quantitative formation of the enolate, with no traces of the base which formed it (which could also behave as a nucleophile); LDA works here as well. Doubly stabilized enolates can be generated with very weak bases (and nucleophiles) so the problem of competition between nucleophiles disappears and the substitution of halides goes smoothly. The reaction of enolates with carbonyl groups is quite general; the only complication occurs if the enolate is reacting with a different ketone, aldehyde, etc. whose conjugate base is similar in energy; proton exchange can occur and the two different enolates can each react with two carbonyls to give four products! Arghhh!
Esters can also be converted to enolates; to avoid changing the identity of the alcohol part of the ester, it is essential to use the conjugate base of that alcohol to make the enolate, e.g. for a Claisen condensation.
Amide Ions
Amide (NH2-) is the conjugate base of ammonia, and is a much stronger base than hydroxide or alkoxide. It can be bought, or made by adding sodium to liquid ammonia. Initially the solution is blue because of intermediate species stabilizing the electron as it is being transferred, and this solution can be used as a reducing agent, e.g. to reduce alkynes to alkenes. It reacts rapidly and quantitatively with aldehydes and ketones to make the enolate and with alkynes to convert them to their conjugate bases (which are also good nucleophiles). With unsymmetrical ketones, lithium di(isopropyl) amide (the conjugate base of propan-2-amine, usually abbreviated LDA), gives enough steric hindrance to ensure that the less substituted enolate is formed.
Hydrides
What is the conjugate acid of "hydride"? Dihydrogen (H2)! Not exactly an acid! But hydrides like LiAlH4 and NaBH4 are bases (as well as reducing agents). Their basic behavior requires great care in working with them. It is usually a good policy to assume that they have reacted to some extent with atmospheric moisture on their travells, especially if the bottle has been opened, and thus that dihydrogen may be emitted from the bottle (first hazard - fire and explosion). If they have reacted with moisture, they will no longer be effective as reducing agents (a different kind of hazard - wasted time). If you ever have to use a hydride reagent, take a small sample and add a little ethanol to it; if you get bubbles, the reagent is still useable (what are the bubbles?). Most chemists use a huge excess, especially of LiAlH4, to ensure that there is actually enough hydride there to do the reduction. LiAlH4 will reduce any carbonyl group, and a lot of things you won't learn about in this course (for example, epoxides to alcohols - remember like other bases it is a nucleophile). But NaBH4 is a little less reactive and thus more selective: it will not reduce carboxylic acids at all and barely touches esters and amides.
Organometallic Compounds
What is the conjugate acid of a Grignard reagent? An alkane! Again, not exactly an acid! It is important to remember that Grignard reagents are very strong bases since they too react with moisture in the air. In fact, it is crucial to the formation of a Grignard reagent in the first place that the apparatus and the other reagents be absolutely free of water; not only will water destroy the Grignard reagent that is formed but it will prevent the reaction starting again. Another situation where the Grignard reagent's basicity may be important is in its reaction with a hindered ketone (especially if the Grignard reagent itself is large); if the reagent cannot react with the carbonyl fast enough it may remove the acidic a proton to form the enolate, destroying the Grignard and accomplishing nothing of great importance.
Grignards can also act as nucleophiles with halides. Since they are made from halides, this reaction, which makes an alkane dimer, could interfere with your synthetic plans for the Grignard reagent. The use of diethyl ether (or THF) as a solvent prevents this reaction, presumably because the ether solvates the Grignard reagent and prevents it getting close to the halide carbon. It is not so easy to prevent this reaction with other metals: adding sodium to an alkyl halide makes some organosodium which then reacts with halide, producing a dimer (Wurtz reaction). Alkyl sodium and lithium compounds react with carbonyl groups similarly to Grignard reagents, but alkyl lithium reagents are a little less reactive and thus more selective.
January 1999. Essay by Dr. Linda M. Sweeting, suggested by Justin D. Chandler