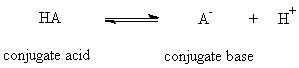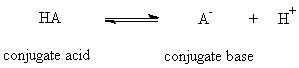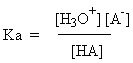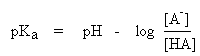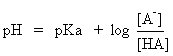Acids / Electrophiles
© Linda M. Sweeting 1999
Acidity and pKa
For a review of pH and pKa and calculations using them, see
the materials in the Chemistry
Tutoring Center. Here is a quick summary. For a generic acid HA, acidity may be expressed as:
Equation 1
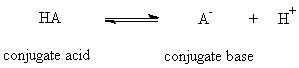
or, more correctly,
Equation 2
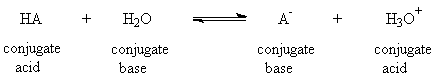
This equation is true no matter what the charge type. For example HA
could be H3O+ or HSO4- and then A- would be H2O or SO42-, respectively. For the acid HA, the Ka and pKa are defined as follows (using
equation 2):
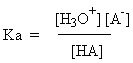
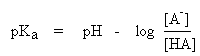
For your chemical edification, either equation is known as the Henderson-Hasselbach
equation; it can be rearranged to predict the pH if the pKa
and concentrations are known (don't forget, though, that either HA or A- can react with water so that the concentrations of material you added may not be the final concentration).
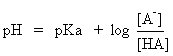
These relationships are based on the assumption that the solutions are dilute and aqueous; as a practical matter dilute means less than 1M.
Dilute weak acids have measurable concentrations of both HA and A-
present. For such weak acids in aqueous
solutions, the proton donor may be H3O+ or the original
acid HA if the acid is serving as a catalyst. A good example of such a weak acid is CH3CO2H. Note that a stronger acid will have a larger Ka and a smaller pKa (pKa may of course be negative, and commonly is for strong acids).
If HA is a strong acid, it will all be converted to A- and H3O+, i.e. completely dissociated in dilute aqueous solution, so that [H+] or [H3O+] = the concentration of HA originally added to the solution. Since no HA remains in the solution, the H-H equation cannot be used (using it would require dividing by zero). Even though HA is a stronger acid than H3O+, the real acid, or proton donor, in aqueous solution is H3O+ since all the HA has been converted to H3O+. H3O+ is the strongest acid that can exist
in dilute aqueous solution. This failure of aqueous solutions to contain stronger acids than H3O+ is a consequence of the equilibria, and is called the "leveling effect".
Often in organic chemistry we are working in non-aqueous media, or in acid
concentrations much higher than 1M, because the reaction needs much stronger
acid for catalysis than H3O+. Under these strong
acid conditions, the basic assumption of pH does not apply, and the activity,
or effective concentration, of the acid is much higher than the molarity,
because the reagent that is transferring the proton is a much stronger
acid than H3O+. A good example of this behavior is
sulfuric acid, whose effective pKa is about -13 (yes, minus
13); the sulfuric acid molecule is a much stronger acid than H3O+
(pKa -1.7) . Such a concentrated solution, although it may contain some water, can hardly said to be an aqueous solution any more - the solvent is the sulfuric acid.
Similarly, the strongest base in aqueous solution
is hydroxide, so very weak acids with very strong conjugate bases cannot be converted completely to their conjugate bases in aqueous solution. To use the strong conjugate bases of very weak acids like ethanol or ammonia (yes, ammonia), you must work in non-aqueous solution as well.
See the essay on bases for more information.
Aqueous Acids and Their Concentrated Forms
H3O+
In dilute aqueous solution, i.e. 1 M or less, where the assumption that concentration reflects activity is correct, the source of the H+ is of no consequence to its activity (page up for more information on aqueous acids). Thus, when acid concentrations are low, we often just write H+ or H3O+ to indicate some strong acid. Such usage is correct if the identity of the acid does not matter. As a practical matter, chloride and nitrate salts are more soluble than sulfate, so HCl and HNO3 are more often used, with HCl getting the edge because it is cheap and a weaker oxidizing agent. H3O+ is often written in an equation as a reagent or catalyst when the identity of the conjugate base of the actual strong acid is unimportant because it does not participate in the reaction.
H3O+ will add the elements of water (H+ and OH-) to an alkene, an alkyne or an epoxide in processes that rely on several chemical equilibria; the products, alcohols and aldehydes or ketones, now contain oxygen but they have not been oxidized since hydrogen was also added. The addition to an alkene can be reversed using very strong concentrated acid (see H2SO4 below). The addition to a carbonyl group, also via acid-base equilibria, is almost always followed by a subsequent elimination to regenerate the carbonyl group; with esters and amides the OH remains and the amine or alcohol is lost since carboxylic acids are more stable than amides or esters. (Note: addition + elimination = substitution.) These hydrolysis reactions of the carboxylic acid family require heat. Some members of the ketone family can also be hydrolyzed. Imines, acetals and hemiacetals are all readily converted back to the ketones (or aldehydes) in aqueous acid. No heat is needed unless the imine or acetal is very stable, as it would be for a 2,4-dinitrohydrazone. Sugar hemiacetals are more stable than the open chain compound so the equilibrium leaves hemiacetal as the major species. When they come to equilibrium they give an equilibrium mixture of diastereomers (anomers); if you started with one diastereomer, the optical rotation changes during the equilibration, a process called mutarotation.
H2SO4
Concentrated (95%) sulfuric acid is a high-boiling, syrupy liquid. (For perspective, calculate the mole percent of water and the molarity - its density is 1.84 g/mL). It is slowly corrosive to just about anything and acts as strong dehydrating agent for a wide variety of materials. See alcohol dehydration below. A spectacular demonstration of its ability to dehydrate can be performed by adding a few drops to a couple of grams of any sugar (sugars are poly-alcohols) in a large beaker - slowly the material gets hot, foams and turns black: the water is removed leaving mostly a foam of carbon. Spectacular but dangerous. Concentrated sulfuric acid - even 50% - is far more acidic than dilute aqueous solutions, as the solvent is really sulfuric acid itself. Sulfuric acid undergoes autoprotolysis (it protonates itself) at these concentrations. See below for "fuming sulfuric acid."
HNO3
Concentrated nitric acid is only 69% but it is dangerous because it is a powerful and rapid oxidizing agent. In spite of its power as an oxidizing agent, it is not a strong enough acid to react with aromatic compounds. The addition of the stronger acid sulfuric acid serves to dehydrate the nitric acid to form NO2+, a much stronger Lewis acid, and allow the nitration to occur. The nitration itself results in loss of a hydrogen and thus is an oxidation. Mixing sulfuric and nitric acids allows pure nitric acid to be distilled out of the solution (its boiling point is 83 C) - boy is that stuff, called "fuming nitric acid", corrosive - instant skin death on contact!
HX (HCl, HBr, HI) and Substitutes
The hydrohalic acids are gases and thus the aqueous "concentrated" solutions are not very concentrated; conc HCl, for example, is 35% in water. The high vapor pressure of the gaseous acid over the solution makes these acids very dangerous to work with, because the chances of exposure to the lungs is quite high. They are exceptionally good at corroding metals (my personal hypothesis is that the high vapor pressure is responsible).
Although HX's can be used for substituting alcohols with halide X, all sorts of side reactions occur with tertiary and secondary alcohols (rearrangement and dehydration). Better methods for substitution are: thionyl chloride (SOCl2) or PCl3, and for secondary and tertiary, the Lucas reagent, which is HCl made more acidic and more chloride-rich by a high concentration of the Lewis acid ZnCl2 (the water is now overwhelmed). Conversion of a carboxylic acid to an acid chloride cannot be done with aqueous HCl (the more stable acid is favored), but must be done with anhydrous SOCl2 or PCl3.
HBr and HI can more readily undergo homolytic cleavage (one electron to each atom) than HCl, since the halogens are not so electronegative. Thus, with a little push, e.g. by a peroxy radical that steals an H atom, these two acids can form bromine and iodine atoms, which can undergo free radical chain reactions. This reaction is the reason for the "antimarkovikov" addition of HBr to alkenes. In the upper atmosphere, chlorine atoms too can survive, and both chlorine and bromine atoms are responsible for free radical chain reactions with convert ozone (O3) back to dioxygen, thus destroying the ozone layer which protects the earth from the near ultraviolet light from the sun.
Anhydrous Acids and Lewis Acids
Lewis acids are those which cannot provide a proton to another molecule but clearly react with bases. Like the proton itself, they are clearly in need of electrons. Their love of electrons has resulted in their being called "electrophiles". Although we generally use the term "electrophile" when no H+ is involved, it is also correct to call protic acids "electrophiles"
H2SO4 with SO3 or HNO3
There is enough water in concentrated sulfuric acid to keep it from reacting with the highly unreactive benzene ring. Addition of SO3 converts the last of the water to sulfuric acid, and, either through autoprotolysis or the presence of excess SO3, allows aromatic substitution (addition + elimination) by SO3. This solution can be purchased as "fuming sulfuric acid". Nitric acid is both a weaker acid and a stronger base than sulfuric and thus is protonated by it (hint - write the equation for the reaction) and eventually dehydrated to NO2+, which is a powerful enough Lewis acid to add to the benzene ring (see above). Note that both of these reactions, although initiated by acid, result in loss of a hydrogen and thus are oxidations.
AlCl3 and FeCl3
Aluminum chloride has a central aluminum atom which has only 6 electrons; like boron in the acidic BF3, it is very electron-deficient. It is one of the stronger and more common Lewis acids used in chemistry. In the presence of alkyl halides (tertiary best, of course) or acyl halides, it can remove the halide to generate a carbocation (why are they stable?) and AlCl4-. The carbocation will react with whatever is available, even add to an aromatic ring. With carbons that are less able to support a positive charge, the aluminum chloride just loosens the C-Cl bond enough to make the carbon positive enough to react with the aromatic ring (and to rearrange as if it were a carbocation). Aluminum chloride is a powerful dehydrating agent as well, although not very selective; partially hydrated (to reduce its acidity) it works very well as commercial dehydrating agent - the antiperspirant aluminum chlorhydrate. Ferric chloride is the catalyst of choice for halogenating benzene rings. The reaction proceeds like that of alkyl halides with aluminum chloride, namely polarization to remove a halide to form FeCl4-, and thus generating Cl+. An easy way to do this reaction is just to add powdered iron to the reaction and an excess of halogen; the halogen oxidizes the Fe to FeX3 (where X could be other halogens as well) which then catalyzes the reaction.
ROH2+
All oxygen-containing organic compounds, like alcohols, behave as bases in the presence of concentrated aqueous acids or anhydrous Lewis acids. Depending on what else is present, the temperature and the acid concentration, alcohols can be converted to ethers (one water lost for two molecules of alcohol) or alkenes (one molecule of water lost per molecule of alcohol). When acid and alcohol are added to other oxygen-containing compounds like ketones and acids and esters, they share the proton in equilibrium. The protonation of the oxygen makes the carbonyl carbon more susceptible to attack by nucleophiles in each case; however, any nucleophile that you use must be no more basic than the alcohol or carbonyl compound or it will be protonated instead! If just the carbonyl compound, alcohol and acid are present, ketones and aldehydes undergo addition to produce hemiacetals and acetals, although they are easily converted back to the aldehyde or ketone in the presence of water in most cases. With esters, acids and acid chlorides, the final product of reaction with acid and alcohol is an ester (perhaps a different ester if you start with an ester) via an addition - elimination sequence. See above for the story of alcohols in concentrated sulfuric acid.
January 1999, Revised February 1999. Essay by Dr. Linda M. Sweeting, suggested by Justin D. Chandler