Unsymmetrical, Polar Reagents

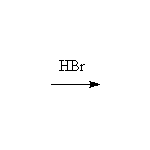
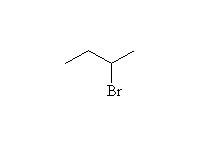
Mechanism! May result in carbocation rearrangements.
McMurry 6.8, 6.9, 7.2, 9.17, Fessenden 10.6, Schmid 7.8 - 7.17

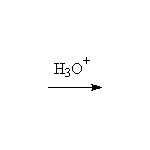

Mechanism! May result in carbocation rearrangements.
McMurry 7.4, Fessenden 10.7, Schmid 7.8 - 7.17
 + H2O
+ H2O
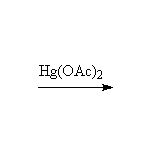
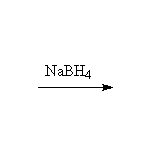
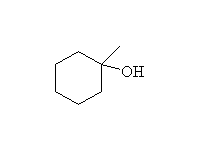
Oxidation, followed by reduction, giving the more substituted alcohol.
Other reagents: acid and water (see above), but these simple addition conditions give rearrangements if a more stable carbocation is possible.
McMurry 7.4, Fessenden 10.8, Schmid 7.14, 8.10

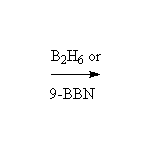
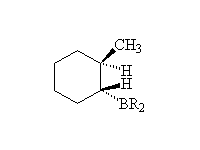

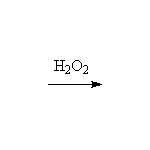
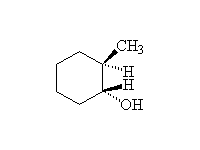
Reduction followed by oxidation, giving the less substituted alcohol.
9-BBN and B2H6 can also be used to make amines and halides.
McMurry , Fessenden 10.8-10.9, Schmid 8.1 - 8.4, 22.11

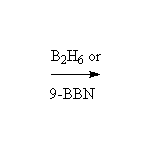


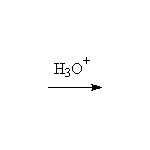

Reduction! BH3 does not exist as such, but dimerizes to form B2H6. Alternatives are available: 9-BBN, BH3 in an ether solvent or complexed to an amine. Click on the blue BBN box for more information.
McMurry , Fessenden 10.8-10.9, Schmid 8.1 - 8.4
Symmetrical Reagents
Reduction
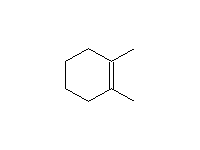
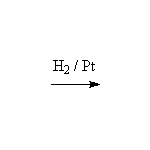
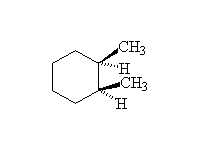
Stereospecific! Surface reaction, complete reduction usually occurs
McMurry 7.7 , Fessenden 10.12B, Schmid 8.11
Oxidation

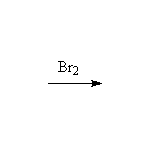

Mechanism! Stereospecific!
Note that I2 adds like Br2; Cl2 adds too but is not stereospecific as it is too electronegative and too small to form a "chloronium" ion. F2 reacts explosively.
McMurry 7.2, 10.3, Fessenden 10.10, Schmid 8.8

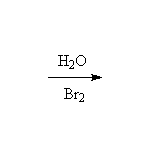
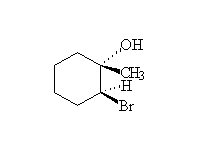
Mechanism! Stereospecific!
McMurry 7.3, Fessenden 10.10D, Schmid 8.9

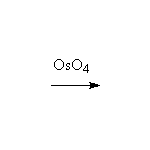
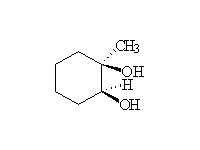
Sterospecific! Oxidation. Osmium can be reoxidized in the reaction with hydrogen peroxide.
Other reagents: cold basic KMnO4 will also accomplish this seterospecific oxidation, but on heating cleaves the double bond like ozone (Schmid 14.3)
McMurry 7.8, Fessenden 10.13, Schmid 8.5

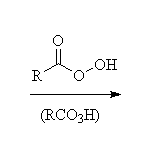
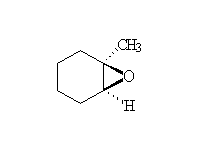 + RCO2H
+ RCO2H
Stereospecific! See ethers for the opening of the epoxide ring.
McMurry 17.4, Fessenden 10.13, Schmid 8.6
Miscellaneous carbon-carbon bond-forming reactions

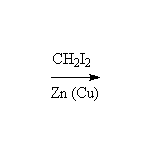
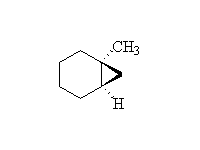 + ZnI2
+ ZnI2
Stereospecific!
A dichlorocyclopropane ring can be made using chloroform and base (e.g. t-butoxide)
McMurry 7.7, Fessenden 10.13, Schmid 8.7
 +
+
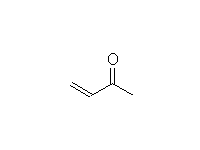

Diels Alder Reaction. Stereospecific! This is a reaction of the alkene and diene, not the C=O; it works best when the alkene is electron-deficient, so the C=O's function is to withdraw electrons
McMurry 14.8, 30.6, Fessenden 16.3, Schmid 19.12 (NOT 19.10 and 19.11)
To reactions of dienes; for reactions alpha to the alkene, see alkane reactions
To synthesis of alkenes or back to the main graphical menu
Last update Nov.1, 1998.