By elimination from alcohols: Dehydration
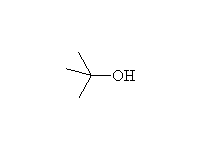
 +
+ 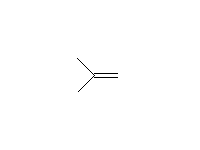
Mechanism!; SN1 and E1; other reagents: HCl, HI
McMurry 5.1, 7.1, 11.10, 11.16, 11.19, 17.7, Fessenden 7.4-7.6, Schmid 11.17, 12.10, 12.11, 12.16
Elimination
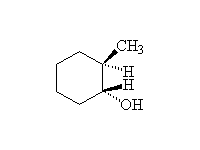
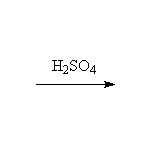
 + H2O
+ H2O
Mechanism!; E1
McMurry 7.1, 11.10, Fessenden 7.6
By elimination from alkyl halides: Dehydrohalogenation

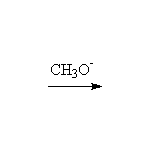
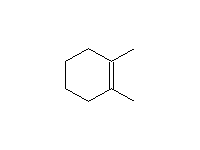
Mechanism! E2, Stereospecific
McMurry 11.12, 11.15, Fessenden 5.7-5.8, Schmid 12.12 - 12.15
By reduction
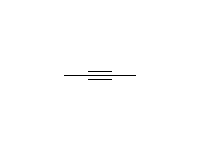
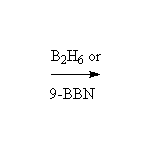
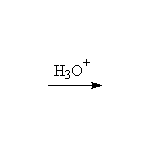
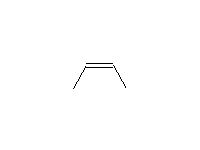
Stereospecific!
McMurry 8.5, Fessenden 10.9, Schmid 9.8
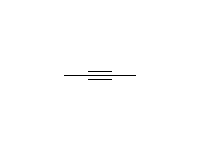
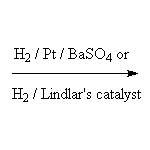

Stereospecific! For other reagents, click on the arrow above.
McMurry 6.7, 8.6, 8.10, Fessenden 10.12B, Schmid 9.8
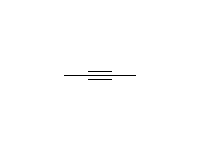
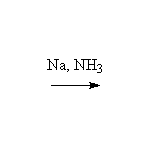
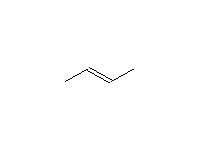
Stereospecific!
McMurry 8.6, Fessenden 10.12B, Schmid 9.8
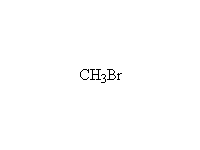
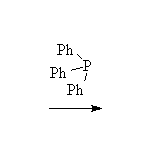
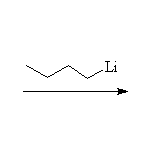
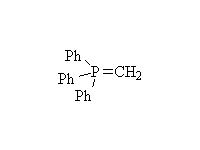

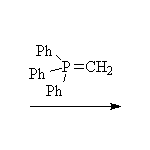

The Wittig reaction; works best with less substituted halides, aldehydes and ketones.
Fessenden 13.4D, Schmid 14.16, 14.17
Miscellanea
 +
+
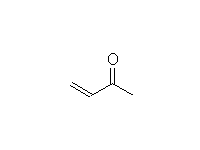

Diels Alder Reaction.
Stereospecific!
This is a reaction of the alkene and diene, not the C=O; it works best when the alkene is electron-deficient, so the C=O's function is to withdraw electrons
McMurry 14.8, 14.9, Fessenden 16.3, Schmid 19.12 (NOT 19.10 and 19.11)
Diene synthesis can be accomplished by all of the reactions above (except the Diels-Alder); where the reaction provides a choice, at equilibrium the conjugated (1,3) diene is more stable than the unconjugated diene or the alkyne.
To reactions of alkenes or back to the main graphical menu
Last update Nov. 1, 1998