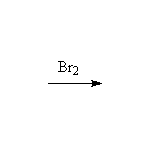Reactions of Alkanes
You may use these summaries and problems but you may NOT download them for use at another site, nor may you charge for access to them. Copyright Linda M. Sweeting 1997
Alkanes are generally pretty unreactive.
To synthesis of alkanes or back to the main graphical reactions menu

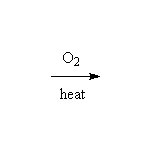 7 CO2 + 14 H2O
7 CO2 + 14 H2O
Combustion occurs for all organic compounds except those that are perchlorinated or perfluorinated. The reaction can be used to determine simple formula or heat of combustion (from which can be determined relative stability).
McMurry 4.4, Schmid 2.10, 7.5

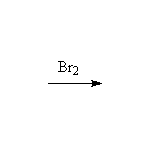
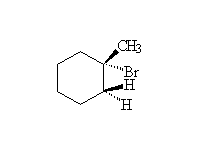
Mechanism! Free radical reaction produces the most substituted halide in the highest concentration. Reaction accelerated by heat and/or light.
McMurry 7.5, Fessenden 6.1-6.6, Schmid 18.1 - 18.4,
Activated alkanes
An adjacent carbon-carbon double bond or aromatic ring stabilizes the intermediate radicals (allyl and benzyl) and makes reaction at these sites easier than tertiary.
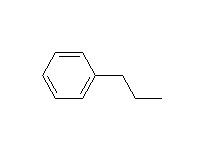
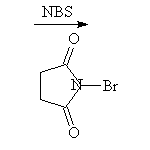

McMurry 10.4, Schmid 19.3, 20.9A, Fessenden 6.5

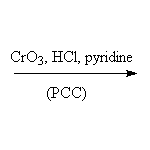
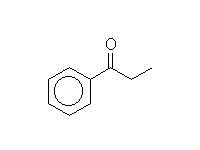
Other reagents:
CrO3 + HOAc, CrO3 + H2SO4 + H2O, and other weakened chromium reagents.
Fessenden 13.7, Schmid 20.9B
Reactions at alkane sites adjacent to carbonyl groups depend on their acidity and are quite different from those of alkanes.
To synthesis of alkanes or back to the graphical reactions menu.

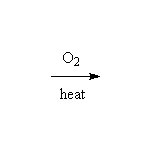 7 CO2 + 14 H2O
7 CO2 + 14 H2O