
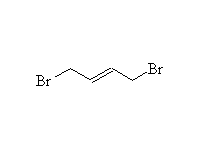 +
+

Mechanism! Not stereospecific.
1,4-addition dominates when at equilibrium, i.e. under thermodynamic control, at room temperature or higher.
1,2-addition dominates when not at equilibrium, i.e. under kinetic control, below 0oC.
McMurry 14.5, Fessenden 16.2A, Schmid 19.8 - 19.9

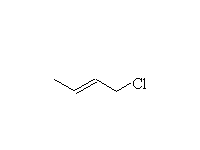 +
+
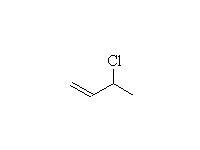
Mechanism!
1,4-addition dominates when at equilibrium, i.e. under thermodynamic control, at room temperature or higher.
1,2-addition dominates when not at equilibrium, i.e. under kinetic control, below 0oC.
Other acids react the same way to give alcohols, bromides, etc.
McMurry 14.5, Fessenden 16.2A, Schmid 19.8 - 19.9
 +
+
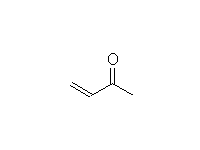

Diels Alder Reaction. Stereospecific! This is a reaction of the alkene and diene, not the C=O; it works best when the alkene is electron-deficient, so the C=O's function is to withdraw electrons
McMurry 14.8, Fessenden 16.3A-C, Schmid 19.12 (NOT 19.10 and 19.11)
Other reactions of alkenes also occur. Hydrogenation is not readily controllable as the original conjugated diene is less reactive than the alkene product.
To reactions of alkenes or back to the main graphical reaction menu.
Last update Feb 10, 1999