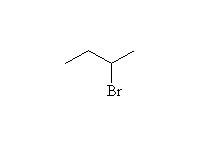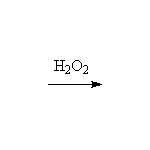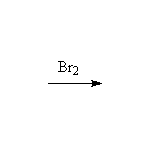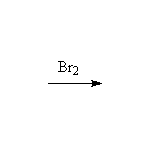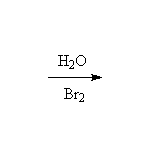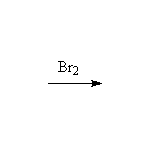Synthesis of Alkyl Halides
You may use these summaries and problems but you may NOT download them for use at another site, nor may you charge for access to them. Copyright Linda M. Sweeting 1997
To alkyl halide reactions or synthesis of aromatic (aryl) halides or back to the main graphical reactions menu
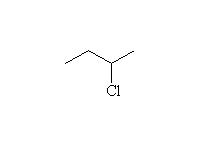
From Alcohols: Nucleophilic Substitution

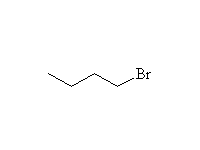
Mechanism!; SN2; other reagents HCl, HI, H2SO4
McMurry 10.7, Fessenden 7.4-7.6, Schmid 11.17, 12.9
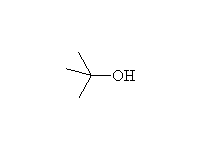

 +
+ 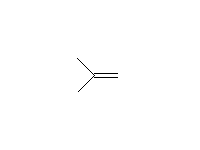
Mechanism!; SN1; other reagents HCl, HI
McMurry 7.1, 10.7, 11.9, Fessenden 7.4-7.6, Schmid 11.17, 12.10, 12.16,

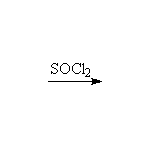
Intermediate is ROSOCl, the half-ester; the reaction is stereospecific.
The reaction of PCl3 and PBr3 is similar; no rearrangements.
McMurry 10.7, Fessenden 7.5, Schmid 11.17,
From Alkenes by Addition


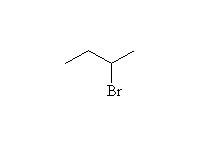
Mechanism! May result in carbocation rearrangements.
McMurry 6.8, 6.9, Fessenden 10.6, Schmid 7.8 - 7.17,

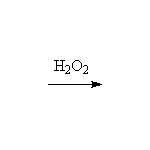


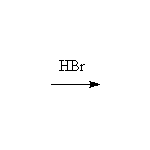
Mechanism! Note that peroxides need to be present at the same time as the HBr for this free-radical anti-Markovnikov addition.
McMurry 5.1, 5.6, 7.5, Fessenden 10.6, Schmid 18.5 - 18.6

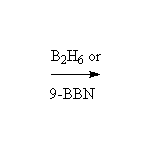
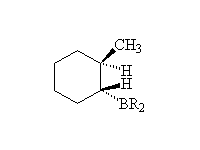


Mc Murry 7.5, Fessenden 10.6, Schmid 8.1 - 8.4


Mechanism! Stereospecific!
Note that I2 adds like Br2; Cl2 adds too but is not stereospecific as it is too electronegative and too small to form a "chloronium" ion. F2 reacts explosively.
McMurry 10.10, Fessenden 10.9, Schmid 8.8

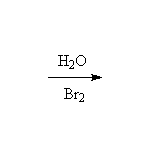
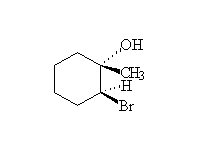
Mechanism! Stereospecific!
McMurry 7.3, Fessenden 10.10, Schmid 8.9,
From Alkanes

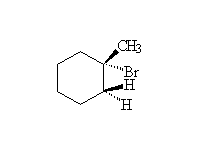
Mechanism! Free radical reaction produces the most substituted halide in the highest concentration. Reaction accelerated by heat and/or light.
McMurry 5.1, 10.4, Fessenden 6.1-6.6, Schmid 18.1 - 18.4,
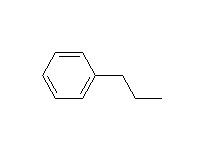
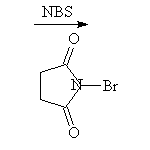

An adjacent carbon-carbon double bond or aromatic ring stabilizes the intermediate radicals (allyl and benzyl) and makes reaction at these sites easier than tertiary.
McMurry 10.5, 10.6, Fessenden 6.5, Schmid 19.3, 20.9A,
To alkyl halide reactions or synthesis of aromatic (aryl) halides or back to the main graphical reactions menu





 +
+