Halogenation
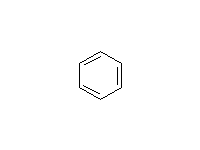
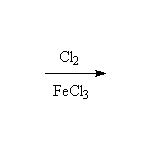
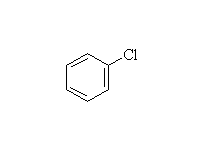
Br2 and FeBr3 will make bromobenzene, and Fe may be used instead of FeX3.
McMurry 16.1 Fessenden 11.8A, Schmid 21.5
Mechanism!
Nitration

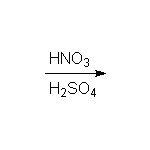
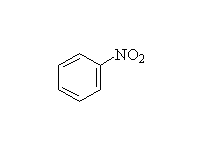
McMurry 16.2, Fessenden 11.8C, Schmid 21.6
Mechanism!
Sulfonation


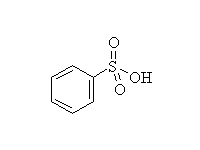
McMurry 16.2, Fessenden 11.8F, Schmid 21.7
Mechanism! Note that sulfonation is the only reversible aromatic substitution. Moreover, fusing arylsulfonic acids with sodium hydroxide (that means melted pure NaOH!) will convert the sulfonic acid to a phenol.
Alkylation - the Friedel-Crafts Reaction
 +
+
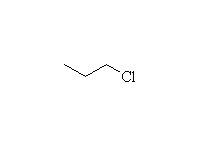
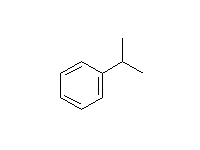 +
+ 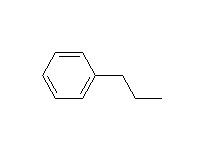
McMurry 16.2, Fessenden 11.8D, Schmid 21.8
Mechanism!
Acylation - Friedel-Crafts
 +
+
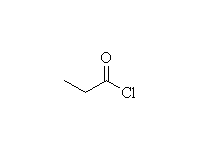

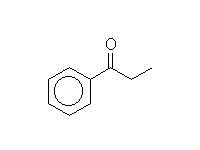
McMurry 16.3, Fessenden 11.8E, Schmid 21.9
Mechanism!
To reactions of ketones (reduction to CH2 is in Schmid 21.10)
If There Is Already a Substituent, You Need to Know the Effect of Substituents on Aromatic Substitution:
The Table Below is in Order of Decreasing Reactivity of Ring Containing the Substituent
| Substituent | Position of Reaction |
log(rate relative to H) |
|---|---|---|
| NR2 | o, p | 1.3 |
| OH, OR | o, p | 0.9 |
| R | o, p | 0.3 |
| Ar | o, p | 0.2 |
| F | o, p | 0.1 |
| H | "o, p" | 0 |
| NHCOR | o, p | -0.1 |
| Cl, Br, I | o, p | -0.1 |
| CO2H | m | -0.4 |
| COR, CO2R | m | -0.5 |
| CX3 | m | -0.5 |
| SO3H | m | -0.6 |
| CN | m | -0.7 |
| NO2 | m | -0.8 |
| NR3+ | m | -0.9 |
McMurry 16.3-16.9 Fessenden 11.9, Schmid 21.11
Mechanisms!
Some notes on multiple substitutions:
Nucleophilic substitution of aromatic rings is possible, but especially if they are electron-deficient or a very strong base is used.
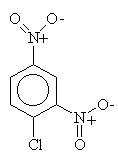
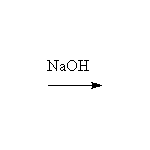
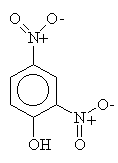
McMurry 16.8, Fessenden 12.4, Schmid 23.1-23.2
Back to the reactions menu