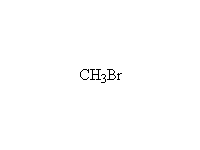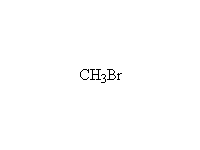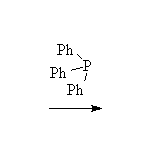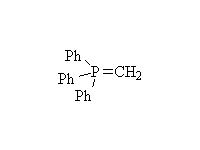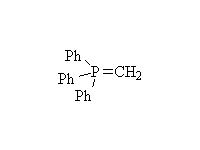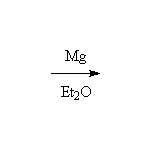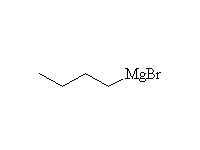Reactions of Alkyl Halides
You may use these summaries and problems but you may NOT download
them for use at another site, nor may you charge for access to them. Copyright Linda M. Sweeting 1997
To synthesis of alkyl halides or back to
the main graphical menu
Nucleophilic Substitution
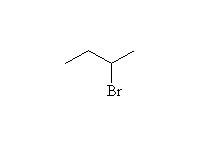

Mechanism! Stereospecific SN2 reaction.
Will be SN1 for tertiary halides (carboations, rearrangements)
Competition also from E2 elimination in non-aqueous solvents
McMurry 11.10, 16.8, Fessenden 5.4, 5.5, 5.10, Schmid 12.1, 12.2,
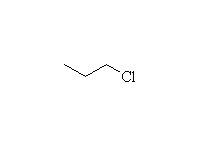


Mechanism! SN2 reaction.
Note that more substituted halides will undergo the E2 elimination
instead.
McMurry 11.14, 11.15, 18.3, Fessenden 5.3, 8.2, Schmid 13.8C,

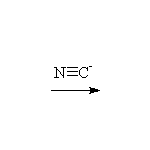
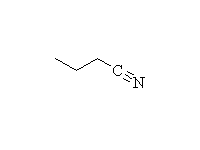
Mechanism! SN2 reaction.
McMurry 11.21, 24.6, Fessenden 5.4, 5.10, Schmid 12.2 - 12.4,
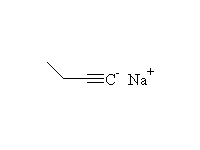 +
+
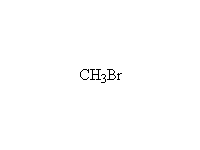
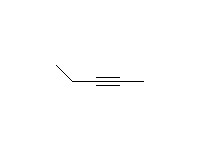
McMurry 8.8, 8.9, 8.10, Schmid 9.10 ,
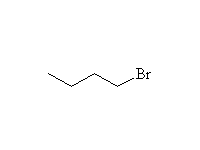

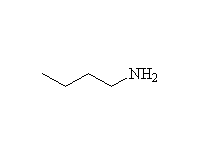
 +
+
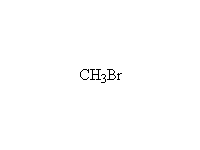
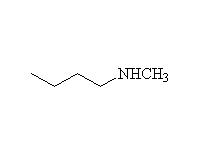

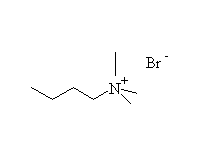
The reaction is hard to control, because the products are at least as
reactive; it works well for making amino acids from halo acids because
the product is the conjugate acid of the amine (the carboxylic acid protonates
it)
Mechanism!
McMurry 24.6, Fessenden 5.10, 18.4, Schmid 22.8,
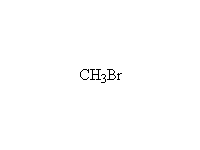
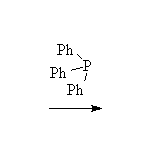
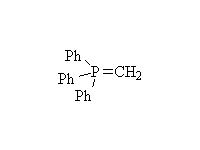

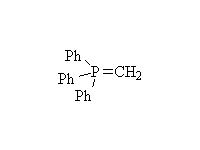

The Wittig reaction; works best with less substituted halides, aldehydes
and ketones.
McMurry 19.12, Fessenden 13.5, Schmid 14.16, 14.17,
Elimination: Dehydrohalogenation

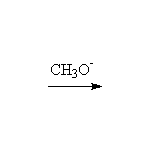
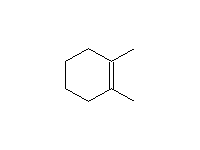
Mechanism! E2, Stereospecific, but may be E1 in polar solvents
McMurry 11.10, 11.13, 11.15, Fessenden 5.7, 5.8, Schmid 12.12 - 12.15 ,
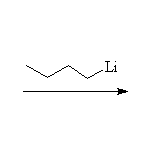
With Metals (Reduction)

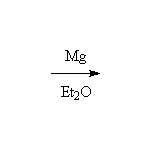
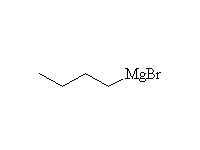
The Grignard reagent
McMurry 10.8, 10.10, 17.6, Fessenden 7.3D, Schmid 11.7,
2 
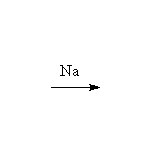
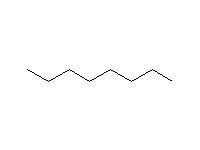
Note that aryl halides do not undergo any of these reactions except
those with metals, e.g. the Grignard reaction. Nucleophilic aromatic substitution
only occurs on rings with strongly electron-withdrawing substituents (Schmid
23.2, 23.3, Mc Murry 10.9).
To synthesis of alkyl halides or back to
the main graphical menu
Last update Feb 10, 1999
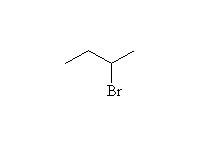





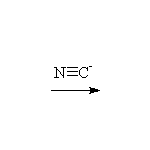
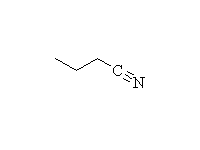
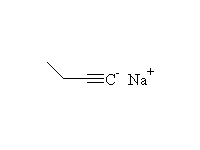 +
+
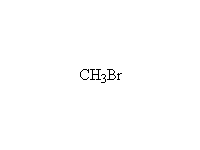




 +
+