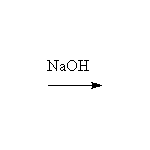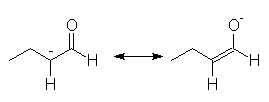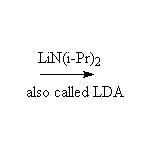Synthesis of Enolates and Enamines
You may use these summaries and problems but you may NOT download them for use at another site, nor may you charge for access to them. Copyright Linda M. Sweeting 1997
The following example reactions are organized by type, with references to text chapters. Links within these summaries (which may show up as boxes around reagents) will provide further information about the reagents and their other reactions. Return to Reaction Summary menu for other functional group choices.

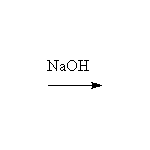
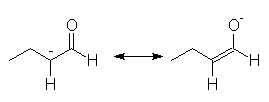
This reaction is a simple acid-base reaction. Enolates may be formed from ketones or aldehydes using a variety of bases, including strong bases such as ethoxide and amide ions. To convert esters to their enolates it is essential to use the conjugate base of the alcohol from which they are formed to prevent hydrolysis or ester exchange. Enolates react very quickly with bromine or other carbonyl compounds

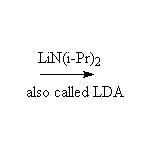
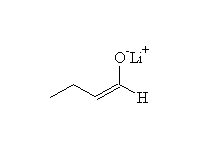
Stable lithium enolate, less substituted if an unsymmetrical ketone
Other reagents: sodium hydroxide or alkoxides with also make enolates (see aldol condensations, for example). However, the acid-base equilibrium is well-balanced and thus the catalyst and both enolates are present if an unsymmetrical ketone is used and any of these may react, especially with a halide.
Other substrates: For esters, the base catalyst must be the conjugate base of the alcohol part of the ester. 1,3-dicarbonyl compounds are more acidic (in fact they existpredominantly in the enol form), so very weak bases can be used to convert them to their enolates.
McMurry 22.5, Fessenden 17.4B, Schmid 17.11


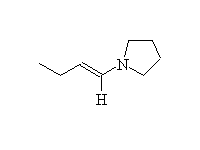
An enamine
McMurry 23.12, Fessenden 17.5, Schmid 17.14
To other reactions of aldehydes and ketones, and esters or back to the main Reactions menu.
Last update February 1999