Nucleophilic Substitution by Enolates and Enamines
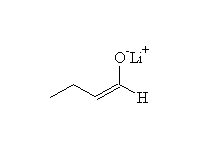
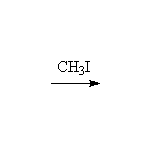
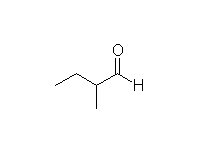
Mechanism!
Alkylation of an enolate is an SN2 reaction
McMurry 22.5, Fessenden 17.4B, Schmid 17.14
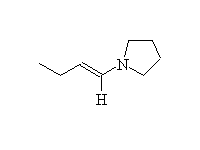
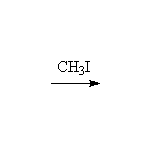

Alkylation of an enamine, plus hydrolysis with water
McMurry 24.7, Fessenden 17.5, Schmid 17.14
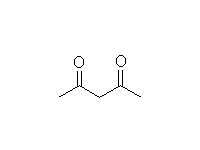 +
+

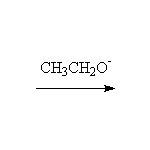
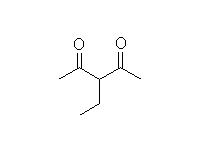
Mechanism!
Other reagents: weaker bases
Decarboxylation upon hydrolysis of 3-ketoesters
McMurry 22.8, Fessenden 17.2B, Schmid 24.7

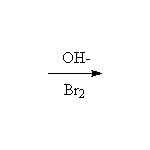
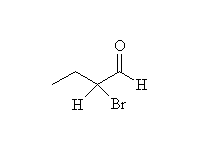
Mechanism!
Bromination of the enolate (think of Br- as a leaving group)
McMurry 22.7, Fessenden 13.10, 17.6, Schmid 17.4
Aldol and Claisen Condensations (Nucleophilic Addition)

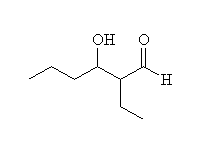 + OH-
+ OH-
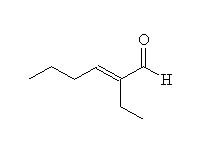 + OH- + H2O
+ OH- + H2O
Mechanism!
Aldol condensation.
McMurry 22.7, Fessenden 17.6, Schmid 17.6

 2
2

Reverse aldol condensation.
McMurry 24.3, Fessenden 17.6, Schmid 17.10
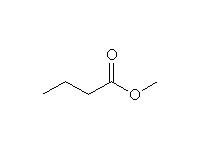
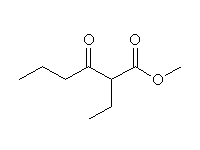 + 2
CH3O-
+ 2
CH3O-
Mechanism!
McMurry 23.6,Fessenden 17.8A, Schmid 17.7, 24.3
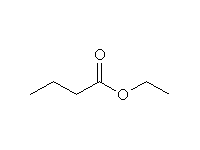 +
+
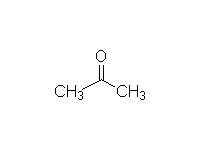
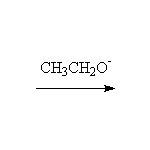
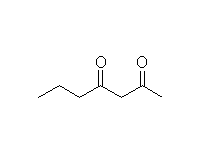
Mechanism!
McMurry 23.2, Fessenden 17.8B, Schmid 17.7, 24.3
Addition to Conjugated Unsaturated Carbonyl Compounds
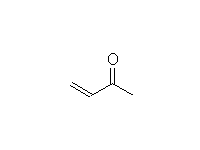
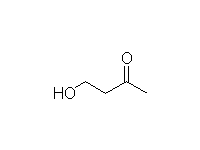
Mechanism!
McMurry 23.4, Fessenden 17.9, Schmid 24.12
 +
+

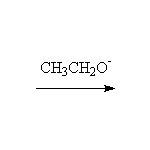
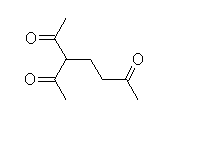
Mechanism!
McMurry 23.11, Fessenden 17.9, Schmid 24.7
To other reactions of aldehydes and ketones, and esters or back to the main Reactions menu.
Last update February 1999