Reactions which break the OH bond
As weak acids

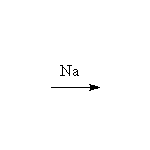
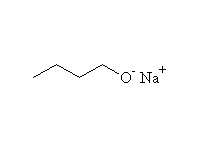 + 1/2 H2
+ 1/2 H2
Other reagents: any base stronger than alkoxide, such as H-, NH2-, CH3MgBr, CH3Li (H2 not formed)
Reference McMurry 17.3, Fessenden 7.2, Schmid 11.15
As nucleophiles with Carboxylic Acids
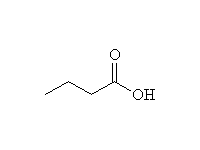
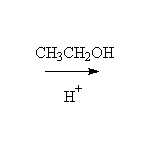
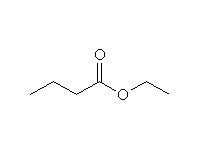 + H2O
+ H2O
Mechanism!
Reference: McMurry 21.3, Fessenden 7.7A, 14.6, Schmid 15.11
To other syntheses of esters
As nucleophiles with Carboxylic Acid Chlorides
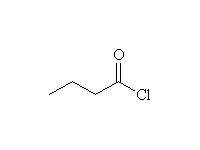
 + HCl
+ HCl
Other reagents: sulfonic acid (sulfonyl) chlorides
Mechanism!
Reference McMurry 21.3, Fessenden 7.7B, 15.3, Schmid 16.5
To other syntheses of esters

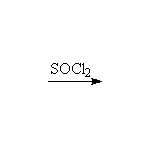
Intermediate is ROSOCl, the half-ester; the reaction is stereospecific.
The reaction of PCl3 and PBr3 is similar; no rearrangements.
McMurry 11.16, 17.7, Fessenden 7.5, Schmid 11.17
As nucleophiles with Esters

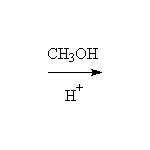
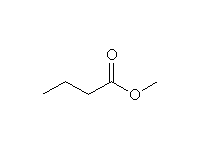 +
+ 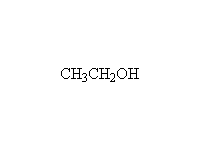
Other reagents: any other alcohols
Mechanism!
Reference Fessenden 15.5C, Schmid 16.9
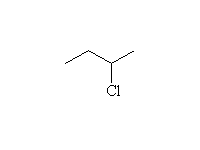
As nucleophiles with sp3 carbon


Mechanism!; SN2 reaction.
Note that more substituted halides will undergo the E2 elimination instead.
McMurry 11.15, 18.3, Fessenden 5.3, Schmid 13.8C
Reactions which break the CO bond
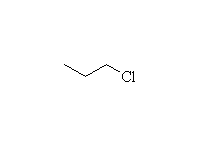
Nucleophilic Substitution

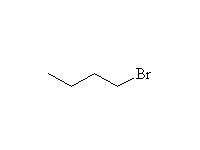
Mechanism!; SN2; other reagents HCl, HI, H2SO4
Reference McMurry 10.7, 17.7, Fessenden 7.4, Schmid 11.17, 12.9
Substitution and Elimination
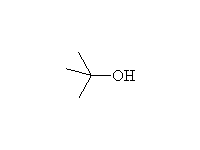

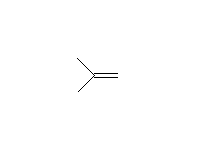
 +
+ 
Mechanism!; SN1 and E1; other reagents HCl, HI
Reference McMurry 10.7, 11.10, 17.7, Fessenden 7.4-7.6, Schmid 11.17, 12.10, 12.11, 12.16
Elimination
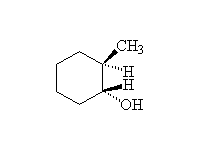
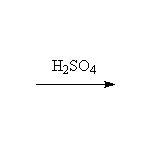
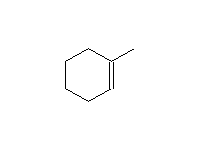 + H2O
+ H2O
Mechanism!: E1; other reagents H3PO4 (acid must be very concentrated).
McMurry 17.3, 17.7, Fessenden 7.6
Oxidation

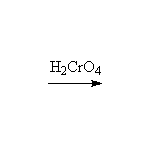
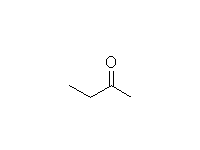
Other reagents: K2Cr2O7 + H2SO4 or CrO3 + H2SO4 or KMnO4 + OH- or KMnO4 + H3O+
Reference McMurry 17.8, Fessenden 7.8, Schmid 11.18

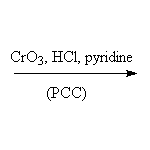

Note that all aqueous chromium reagents and permanganate oxidize primary aldehydes to carboxylic acids and cannot be used for this reaction; selective reagents are needed.
Reference McMurry 17.8, Fessenden 7.8, Schmid 11.18

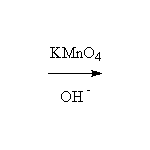
 + MnO2
+ MnO2
Other reagents: H2CrO4 or K2Cr2O7 + H2SO4 or CrO3 + H2SO4
Reference McMurry 17.8, Fessenden 7.8, Schmid 11.18, 15.5
Back to the Graphical Reactions Menu
Last update Nov 1, 1998