Addition / Reduction
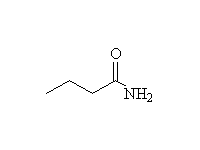
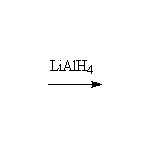
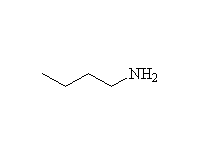
Other reagents: NaBH4 is not a strong enough reducing agent to do this reaction (unless it has help from an acidic reagent).
Nitrile reduction is essentially the same.
McMurry 24.6, 21.7, Fessenden 15.8C, Schmid 16.12, 16.15
To other syntheses of amines
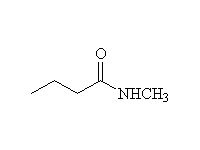
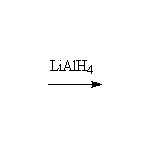
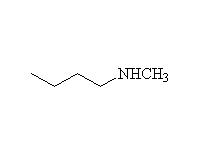
Other reagents: NaBH4 is not a strong enough reducing agent to do this reaction (unless it has help from an acidic reagent).
McMurry 21.7, 24.7, Fessenden 15.8C, Schmid 16.12,
To other syntheses of amines
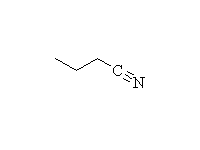
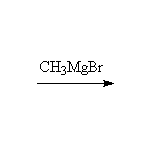
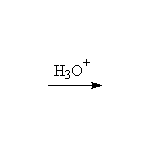
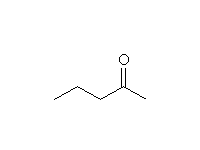
This reaction cannot be done with primary and secondary amides which are acidic enough to destroy the Grignard Reagent. Tertiary amides react like the nitrile shown here but may be sterically hindered from reaction with some Grignards.
This reaction produces an intermediate imine which is hydrolyzed to the ketone on neutralization in water.
Reference McMurry 19.6, 21.6, Schmid 16.15
To other syntheses of ketones
Addition / Elimination: Hydrolysis

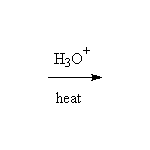
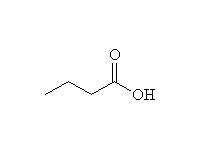 +
+
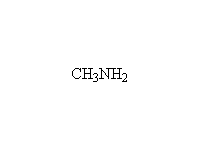
Other reagents: H2O, OH-; primary, secondary and tertiary amides all undergo this reaction.
Nitrile hydrolysis is essentially the same; in fact, amides are intermediates in nitrile hydrolysis.
McMurry 21.6, Fessenden 15.8C, Schmid 15.11, 15.12
To other syntheses of carboxylic acids
To the main graphical reactions menu.
Last update February 1999