Simple Additions
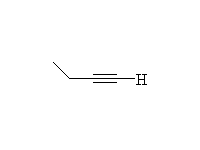
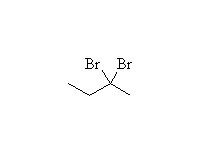
Mechanism!
McMurry 8.4, Fessenden 10.6, Schmid 9.6A
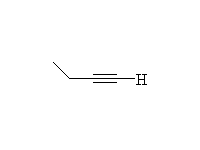
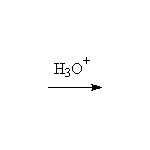
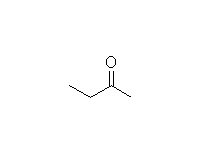
Mechanism! Rearrangement of the enol.
McMurry 8.5, Fessenden 10.7, Schmid 9.7A
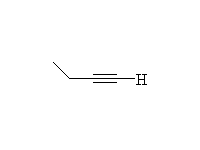 + H2O
+ H2O
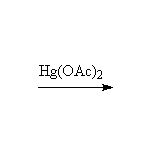
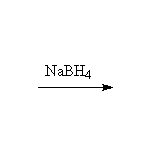

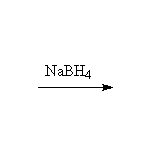

The enol is formed and rearranges to the ketone, which is in turn reduced by the sodium borohydride; sodium borohydride is almost always used in excess because the hydrides react with air and water, making the number of moles uncertain.
McMurry 8.5, 17.15, 19.8, Fessenden 10.8, Schmid 9.7A
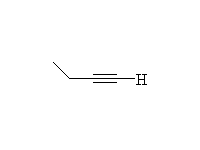
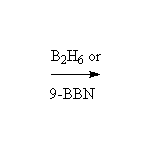
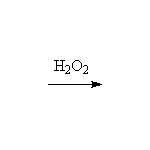

See alkene reactions for details.
McMurry 8.5, Fessenden 10.9, Schmid 9.7B
Reduction
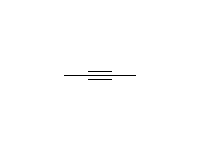
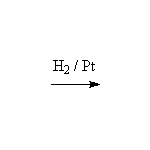
Stereospecific! Surface reaction, complete reduction usually occurs
McMurry 8.6, 8.7, Fessenden 10.12, Schmid 9.8
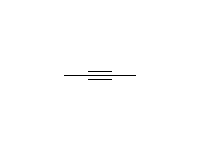
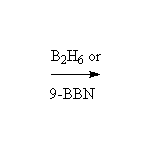
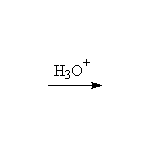
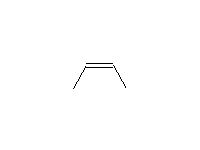
Stereospecific!
McMurry 8.6, Fessenden 10.9, Schmid 9.8
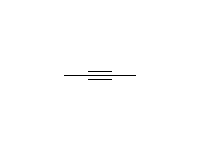
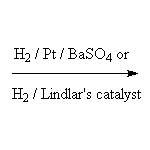

Stereospecific!
McMurry 8.6, Fessenden 10.12, Schmid 9.8
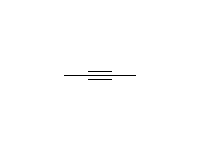
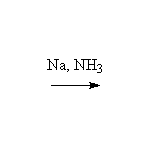
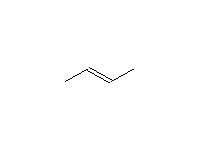
Stereospecific!
McMurry 8.6, Fessenden 10.12B, Schmid 9.8
Oxidation
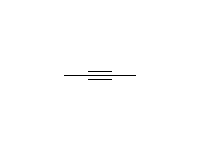
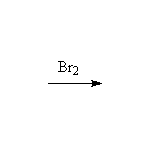
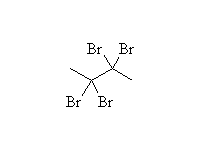
McMurry 8.4, Fessenden 10.5, Schmid 9.6B
As Weak Acids
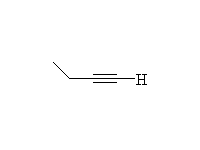
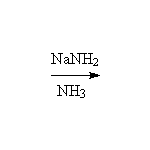
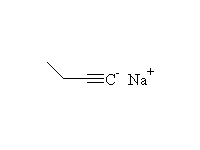
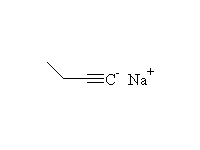 +
+
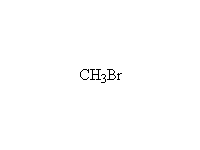
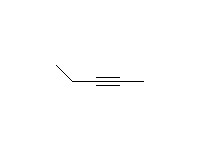
SN2 reaction, stereospecific if halide is secondary
McMurry 8.6, 8.8, 8.9, Fessenden 10.4, Schmid 9.10
To synthesis of alkynes or back to the main graphical menu
Last update Feb. 10, 1999