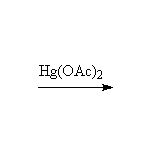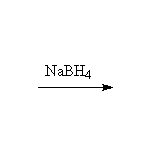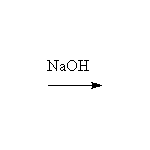Synthesis of Ethers and Epoxides
You may use these summaries and problems but you may NOT download them for use at another site, nor may you charge for access to them. Copyright Linda M. Sweeting 1997
To reactions of ethers or back to the main graphical reactions menu
From alkyl halides and alcohols
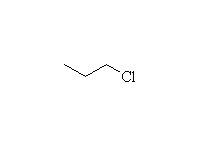


Mechanism!; SN2 reaction, Williamson synthesis
Note that some alkene is also formed, by competition from the E2 reaction; more substituted halides will undergo only the E2 elimination.
McMurry 8.3, 11.5, 11.9, Fessenden 5.3A, 5.10, 8.2B, Schmid 13.8C, 12.2 - 12.5, 12.14
From alkenes
 + CH3OH
+ CH3OH
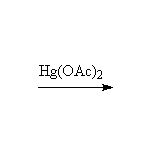
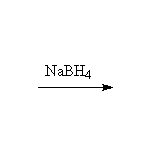
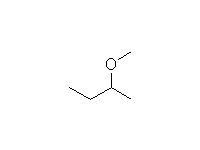
This method is better than the Williamson synthesis if the carbon is not primary.
McMurry 18.4, Fessenden 10.8, Schmid 8.10, 13.8
Epoxides
These strained ethers are very easy to make (from alkenes) but very reactive.

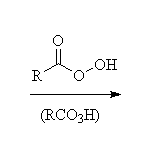
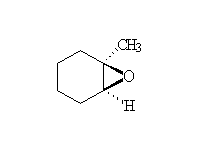
Stereospecific!
McMurry 17.4, 18.7, Fessenden 10.13, Schmid 13.10, 8.6
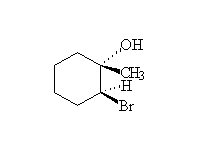
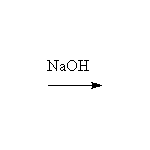

Mechanism! Stereospecific!
McMurry 18.7, Fessenden 8.3, Schmid 13.10, 8.9
To reactions of ethers or back to the main graphical reactions menu
Last update Feb. 1, 1999



 + CH3OH
+ CH3OH