Except for acid-base reactions, products are shown after neutralization in aqueous solution.
To reactions or syntheses of carboxylic acids.

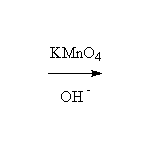
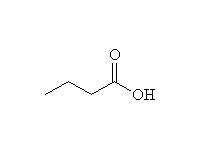 + MnO2
+ MnO2
McMurry17.8, 19.3, Fessenden 7.8C, Schmid 11.18, 15.5

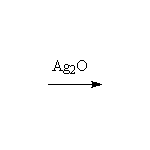
 + Ag
+ Ag
McMurry 17.8, 19.3, 20.6, Fessenden 13.7, Schmid 14.18, 15.5

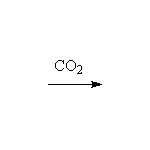
 + MgBrOH
+ MgBrOH
McMurry 20.6, Fessenden 14.3, Schmid 15.6

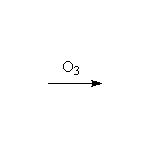
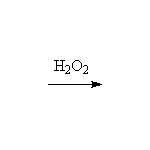

McMurry 7.8, 8.7, Fessenden 10.13B, Schmid 15.5, 14.6

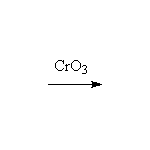

McMurry 16.10, 20.6, Fessenden 7.8C, Schmid 15.5

 + H2O
+ H2O
McMurry 20.3, Fessenden 14.5, Schmid 15.8

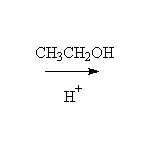
 + H2O
+ H2O
McMurry 21.3, Fessenden 14.6, Schmid 15.11

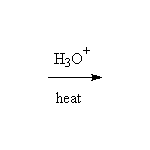
 + CH3CH2OH
+ CH3CH2OH
McMurry 21.5, Fessenden 15.5C, Schmid 15.11, 15.12

 +
+

McMurry 21.7, Fessenden 15.8C, Schmid 15.11, 15.12

 +
+

McMurry 21.7, 21.8, Fessenden 15.11D, Schmid 15.7

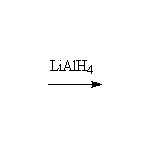

McMurry 17.5, 20.8, Fessenden 14.7, Schmid 15.17

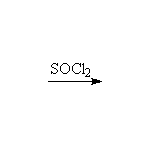
 + SO2 + HCl
+ SO2 + HCl
McMurry 21.3, Fessenden 15.3B, Schmid 16.5

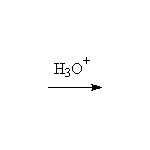
 + HCl
+ HCl
McMurry 21.4, Fessenden 15.3C, Schmid 16.5
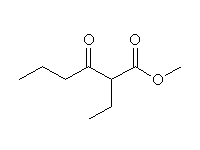
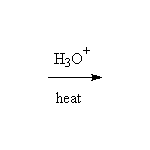
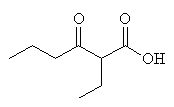

McMurry 21.3, Fessenden 14.8C, Schmid 24.6

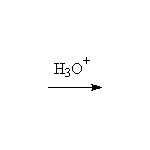 2
2

McMurry 21.5, Fessenden 15.5C, Schmid 16.9
To reactions of carboxylic acids
To the main graphical reactions menu.