In collaboration with Dr. Sweeting
Research into the Phenomenon of Triboluminescence
Triboluminescence is the emission of light by solids when they are fractured; by far the most common example is that of WintOGreen Lifesavers (TM). To see triboluminescence for yourself, take some sugar cubes, some WintOGreen Lifesavers and some rolls of adhesive tape and a friend into a room or closet with no windows. After about 10 minutes (the half-life for dark adaptation), try to crack the Lifesavers with your teeth while keeping your mouth open; if you have managed not to get them too wet, you will see bright flashes of bluish-green light. Now try striking the sugar cubes against each other as if you were striking a match. Last, but not least, pull the tape from the roll, observe, stick it back down again and try again. Note that the rubbery adhesive produces the light in the last case, and is clearly not a solid; the adhesive failure emission is an example of triboluminescence or fractoluminescence with elastic and plastic deformation.
My research is directed toward understanding this phenomenon in crystals. Current theory, supported by spectroscopic, crystallographic, and other evidence, is that charge is separated upon fracture and that the light is produced upon discharge, or recombination of the separated charges. Earlier work, done in collaboration with TSU undergraduates, shows that most organic materials give a perfectly normal emission (fluorescence) spectrum that provides no evidence of the source of the energy, except in a few cases. For crystals to maintain a charge and electric field sufficient for a discharge, theory suggests that their crystals must lack symmetry and they must be poor conductors. Yet many do not have the dissymmetry to permit charge separation in the first place; some may gain their dissymmetry by the presence of impurities. So the research projects below are designed to unravel the dependence of the phenomenon on crystal structure and a variety of related problems.
Suggested projects:
Back to Dr. Sweeting's home page
Stereochemistry of Hemiacetal Formation Studied by Nuclear Magnetic Resonance
This project is currently on hold.

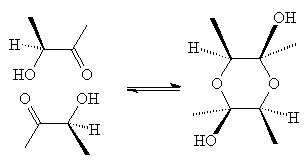
Molecules of aldehydes and ketones with an OH
on the next carbon react with each other to form cyclic hemiacetals. This
reaction is exactly the same as that occurring in sugars, except that a
stable 6-membered ring can be formed with most sugars by a single hemiacetal
linkage and these compounds need two. This reaction is important for the
light it might shed on sugar conformations and on the natural state of
common hydroxyketones observed in biochemistry.
As you can see in the sketch above, the one chiral center of each alcohol gives 4 in the center; with a little effort, you can determine that there are 10 possible stereoisomers formed by dimerization of racemic 3-hydroxy-2-butanone, of which three would be invisible by NMR because they are enantiomers of three others (assuming only one chair conformation is observed for each).
The proton NMR spectrum of this compound in deuterated acetonitrile shows the presence of two compounds, in a ratio of about 5:1, with possible traces of others. One of the compounds is probably the dimer with all the methyl groups equatorial. The 13C NMR show the major component to be the monomer, so the system is not nearly as complex as it might have been. Whew! The concentration of monomer is likely to be sensitive to solvent. As a general rule, C-13 NMR spectra are more readily predicted with adequate accuracy than H-1 NMR spectra and we will probably obtain both spectra routinely.
To understand this equilibrium, we need to do the following:
Back to Dr. Sweeting's home page
Fluorescence Experiment for Organic, Analytical and Biochemistry
Of the 85% of the students taking introductory chemistry courses at Towson State University who are biology majors, many will use fluorescence labelling in their career to map and quantitate proteins and nucleic acids, both generically and selectively. In the current curriculum, biology majors are not exposed to the physical basis of fluorescence and phosphorescence as part of their formal undergraduate training. I propose to develop a set of experiments, in collaboration with students and faculty at Towson State University, to introduce both theory and application of fluorescence in the courses that both biology and chemiatry majors take.
The first experiment in the set will be the synthesis in Organic Chemistry of several fluorescent compounds with different emission characteristics and different labeling potential. Subsequent experiments in Biochemistry laboratory may examine changes in the fluorescence intensity and color of the student-made compounds in the presence of isolated nucleic acids and proteins. Students in Analytical Chemistry and advanced laboratory courses may use these fluorescent dyes for a Beer's law study and, depending on their properties, to examine the effect of pH, solvent, concentration, etc. on fluorescence spectra. The student-made compounds may also be used as fluorescent labels in Biology courses, such as Physiology, in addition to more selective purchased materials.
Synthetic methods will be developed for several fluorescent probes that can be made on a micro scale in good yield from inexpensive starting materials by novices, that are not overly toxic, and would be useful in at least some of the other courses I have described. Ideally, the synthetic methods for all would be similar, not overly exotic (the reactions should be in standard organic chemistry texts), and of course not proprietary.
Student collaborators in the development of these methods will:
Webpage for Organic Chemistry
Back to Dr. Sweeting's home page
Last revised June 2001