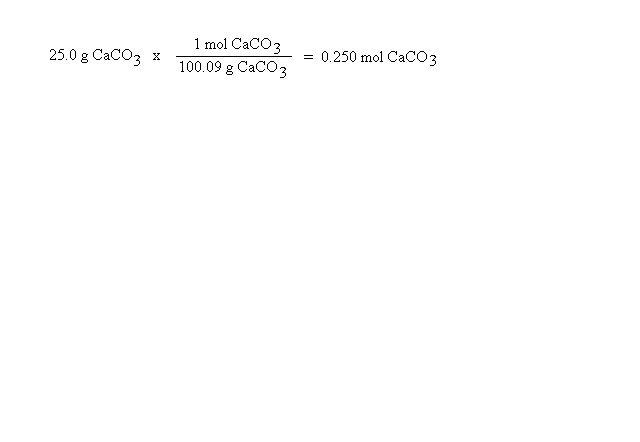
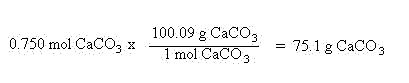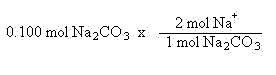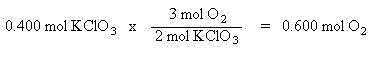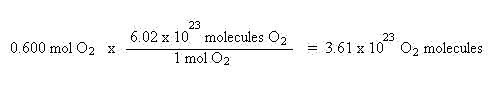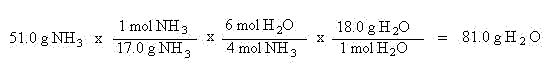Suppose you had 0.100 mol of sodium carbonate and wanted to know how many mols of sodium ions are present. The chemical formula for sodium carbonate is Na2CO3. This means one mole of sodium carbonate contains 2 mols of sodium, 1 mol of carbon and 3 mols of oxygen. In this case, we need to compare the mols of sodium carbonate and mols of sodium ions. Again, we will let the units help us set up the problem:
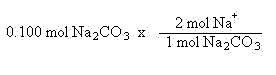
Notice how the units cancel. Also, in this case, we were able to due a stoichiometric comparison within the same compound. In other cases, a reaction may be involved and a comparison of two different compounds is necessary. Given a balanced chemical reaction, the stoichiometric coefficients relate the mols reactants and products. Let's look at a typical reaction:
2 KClO3  2 KCl + 3 O2
2 KCl + 3 O2
In this reaction, 2 mols of potassium chlorate (KClO3) will decompose into 2 mols of potassium chloride (KCl) and 3 mols of oxygen (O2). If you were given 0.400 mol of KClO3, and wanted to know how many mols of O2 will form, use the stoichiometric coefficients to set up a mol ratio in which mols of KClO3 will cancel and mols of O2 remain:
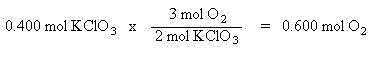
From mols you may also find the number of particles by using Avogadro's number. These particles may be atoms, ions, or molecules. The key word here is particles. That should tip you off that Avogadro's number will be used. Avogadro's number, 6.02 x 1023, represents how many particles are found in 1 mol, so it may be used to convert mols of a substance to particles or the number of particles to mols. Using the above example, let's calculate how many molecules of oxygen were formed:
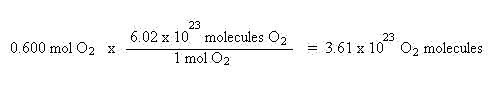
Using the entire strategy
Now that you know how to convert grams to mols, mols A to mols B, and mols to grams, you can utilize the entire strategy, Quantity A --> mols A --> mols B --> Quantity B to work a typical stoichiometry problem encountered in which the theoretical yield of a substance is calculated. The theoretical yield represents the maximum amount of a product that may be produced from a given set of reactants. Here's a typical problem:
If ammonia, NH3, is burned in air, the following reaction takes place:
4 NH3 + 3 O2  2 N2 + 6H2O
2 N2 + 6H2O
Given that you started with 51.0 g of NH3, how many g of water will be produced?
The overall strategy will be to convert the grams of ammonia to mols of ammonia (Quantity A to mols A), then convert the mols of ammonia to mols of water (mols A to mols B), and then finally convert the mols of water to grams of water (mols B to Quantity B):
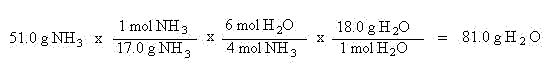
Quantity A  mol A
mol A  mol B
mol B  Quantity B
Quantity B
The first step was to convert Quantity A to mols A -- grams of ammonia to mols of ammonia. Next the mols A was converted to mols B -- mols of ammonia was converted to mols water. Finally, mols B was converted to Quantity B -- mols water was converted to grams water. The 81.0 g H2O produced represents the theoretical yield --the maximum amount of water which can be produced.
In the above problem, we assumed that we had more than enough oxygen to completely consume the ammonia. This assumption may be made when no information is given about the amount of oxygen present during the reaction. In a limiting reagent problem, initial quantities of both reactants are given, and it is not possible to tell which reactant will be used up first. If you need help with limiting reactant problems, please see the specific handout which deals with this topic.
Created With HTML Assistant Pro - 05/11/2001
© Copyrght, 2001, L. Ladon. Permission is granted to use and duplicate these materials for non-profit educational use, under the following conditions: No changes or modifications will be made without written permission from the author. Copyright registration marks and author acknowledgement must be retained intact.