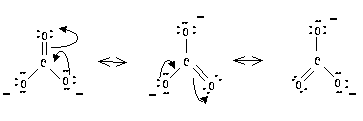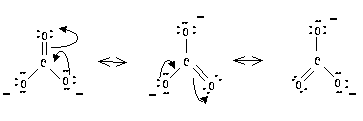DRAWING RESONANCE STRUCTURES
When drawing resonance structures, one must keep in mind that only the electrons are being pushed around. The position of the atoms forming the molecule remain constant. The movement of electrons is shown using arrows. The head of the arrow indicates the final position of an electron pair, the tail of the arrow indicates which electron pair is being relocated. If the head of the arrow points between two atoms, a new bond is being formed. If the head of the arrow is pointing to an atom, a new electron pair appears on that atom. Once one has finished pushing around the electrons, check the formal charges to see if an atom has a charge.
When trying to draw all resonance contributors, use the minimum number of arrows necessary to obtain the structure of each resonance contributor.
Some examples are shown below:
Carbonate ion:
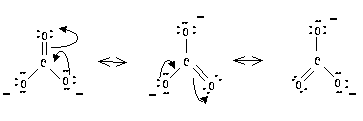
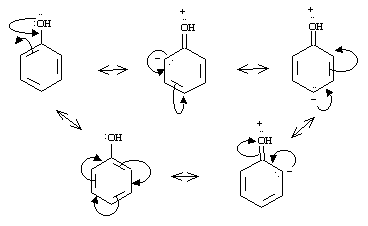
Phenol:
Created With HTML Assistant Pro - 05/11/2001
© Copyrght, 2001, L. Ladon. Permission is granted to use and duplicate these materials for non-profit educational use, under the following conditions: No changes or modifications will be made without written permission from the author. Copyright registration marks and author acknowledgement must be retained intact.