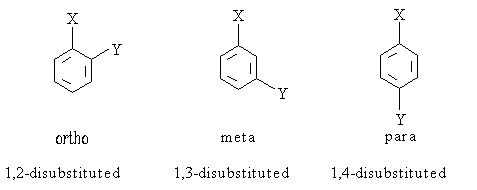
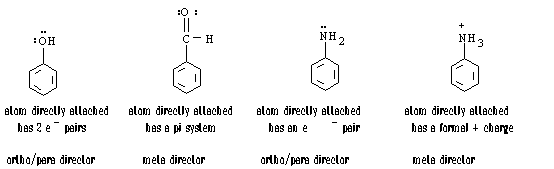
The presence of one substituent on an aromatic ring will dictate the orientation of the second subtituent added to the ring during a reaction. The first substituent will generally be either an ortho/para director or a meta director. Using a few rules, one can predict whether an existing substituent directs the next group ortho/para, or meta:
Ortho/para directors:
Meta directors:
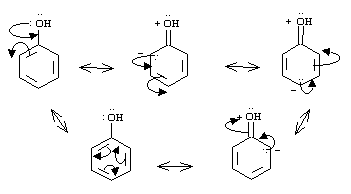
Look at how the formal negative charge is found in the ortho and para positions in the resonance contributors shown above. An electrophile approaching the ring will thus be attracted to these positions, because it is where the electrophile will encounter the highest amount of electron density.
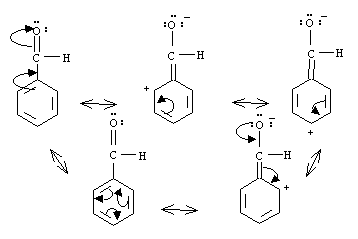
Notice how a formal positive charge is found in the ortho and para positions. An electrophile approaching the ring will be repelled from these positions. Instead, the only ring locations where the electrophile will encounter electron density is at the meta postions.