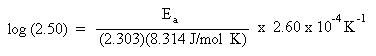In this rate law, the rate of disappearance of NO and O2 is proportional to the concentrations of NO and O2, where k is a rate constant (a proportionality constant). The exponent associated with each concentration term are referred to as the partial order. The sum of the partial orders gives the overall order of the reaction. In the above rate law, the partial order for NO is 2 and is often referred to as 2nd order. The partial order for O2 is 1 and is often refereed to as 1st order. The overall order is 3 (3rd order). It is important to note that the coefficients found in the balanced equation are not related to the exponents found in the rate law.


For the equality to hold true, x must equal 1. This makes the partial order for O2 first order.
The same procedure is used to determine the partial order of NO. Pick two trials in which the concentration of O2 is held constant while the concentration of NO is varied. These criteria are met with Trial 1 and Trial 3. The concentration of O2 is held constant while the concentration of NO has tripled. Under these conditions, the rate of the reaction is increased nine fold. This may be expressed mathematically as:

For the equality to hold true, x must be 2. This makes the partial order for NO second order.
We are now able to write the rate law: Rate = k[NO]2[O2], where k is the rate constant. To solve for the rate constant, choose any experimental trial, and substitute for the rate, and concentrations of NO and O2. Trial 1 data will be arbitrarily chosen in this case:
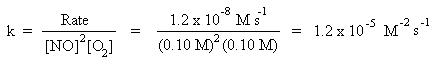
Thus the rate law becomes:
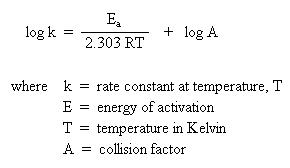
If one were to graph experimental data where log k is along the y-axis and 1/T is along the x-axis, a straight line would result. The slope of the line is Ea/2.303R, and the y-intercept corresponds to log A. By determining the slope of the line mathematically, one could calculate the energy of activation. Similarly, if one extrapolates the line to the y-intercept, one could calculate the collision factor.
Since the slope embodies the energy of activation, a simplified form the Arrhenius equation could be used to determine the energy of activation. One requires knowledge of the rate constants at two different temperatures. Recall that the slope of a line is determined by the change in y over the change in x ("rise" over "run"). With this in mind, one is able to generate the following equation:
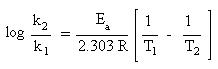
Since the y-intercept, log A, is not part of the slope, it can be ignored. This equation may be used to solve the following problem:
Substitute the rate constants and temperatures into the Arrhenius equation:
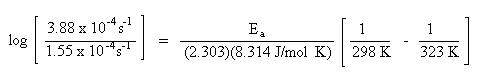
Solve for Ea. Remember to do the arithmetic inside the parenthesis first.