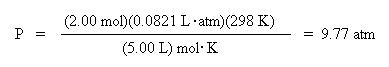Problems Dealing With The Ideal Gas Law

The units of pressure, volume and temperature are dictated by the ideal gas law constant, R. The most common used value for R when dealing with gases is 0.0821 L. atm/mol. K. This unit requires that volume to be expressed in liters, pressure to be expressed in atmospheres, and temperature to be expressed in Kelvin. One thing to keep in mind is that temperature will always be expressed in the Kelvin scale when dealing with any of the gas laws.
Also, the volume must be in liters, not milliliters. Convert as follows:

Now we are ready to insert the known values into the ideal gas law:

Solve for pressure by dividing both sides of the equation by the volume, 0.500 L:
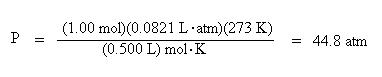
Notice how the units cancel to give atmospheres.
As before, we need to convert temperature from Celsius to Kelvins:
Additionally, we need to convert the pressure units of mm Hg to atmospheres. In one atmosphere of pressure, there are 760 mm Hg:
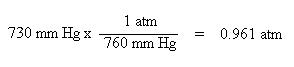
Remember, the reason for these conversions was to make the units consistent with the units of the ideal gas law constant, R.
Substitute the known values for pressure, moles, R, and temperature into the ideal gas law:
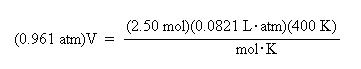
Divide both sides of the equation by the pressure, 0.961 atm, to solve for volume:
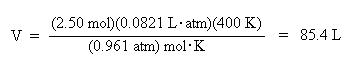
Again, notice how the units cancel to give volume units of liters.
Unit conversions of pressure and volume must be done, first. We see here another pressure unit, torr. There are 760 torr in one atmosphere, and as one can see, the torr pressure unit is the same as the units of mm Hg.
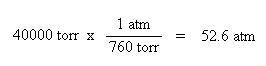
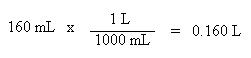
Once again, since the pressure and volume units are now consistent with the units of R, the values may be substituted into the ideal gas law:
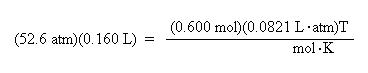
Divide both sides of the equation by the moles, 6.00 mol, and the ideal gas law constant, R,
0.0821 L. atm/mol . K, to solve for temperature:
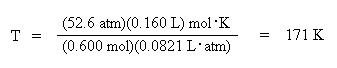
Notice how the units cancel to give temperature in K.
Convert the Kelvin temperature into Celsius:
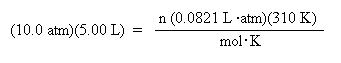
Divide both sides of the equation by the gas law constant, R, and the temperature to solve for n:

Other P,V,T, n Relationships (Empirical Gas Laws)
Since both pressures and volumes are equal to nRT, they are equal to each other:

Substitute the know variables into the equation for Boyle's Law. Make sure that the volume units are consistent. In this case, the volume units are both expressed in liters.
Solve for the new pressure, P2, by dividing both sides of the equation by the new volume, 0.500 L:
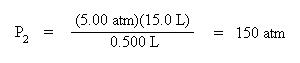
Note that pressure and volume are inversely proportional, so as volume decreases, the pressure increases.
Collect terms. Bring the constants to one side of the equation and the variables to the other side of the equation. Divide both sides of each equation by pressure, P, and by the temperature term, T1 in the first equation and T2 in the second equation. This is Charles' Law:
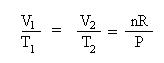

Substitute the known temperatures and volume into the expression for Charles' Law:
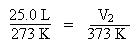
Solve for V2 by multiplying both sides of the equation by T2, 373 K:
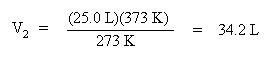
Note how volume and temperature are directly proportional. As the temperature increases, volume increases.
Collect terms. Divide each equation by their respective temperature term and each equation by the volume, V:
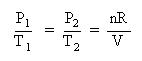
Again, the first step is to convert the temperatures from Celsius to Kelvin:
Set up a table of knowns and unknown:
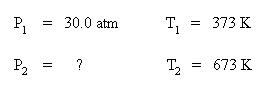
Substitute the known quantities into the Guy-Lussac equation:
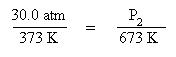
Solve the above expression for the pressure, P2, by multiplying both sides of the equation by the temperature, 673 K:

Notice that pressure and temperature are directly proportional. As the temperature increased, the pressure also increased.
Collect terms. Divide each equation by pressure, P, and divide each equation by their respective mole term:
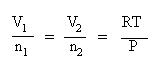
Set up your table of knowns and unknown:
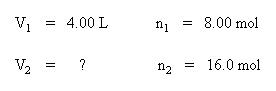
Substitute the known quantities into the equation for Avogadro's Law:
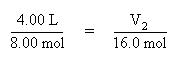
Solve the above equation for the volume, V2, by multiplying both sides of the equation by 16.0 mol:
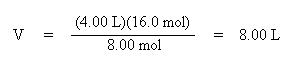
Notice how volume and moles are directly proportional. As the number of moles of gas increased, so did the volume of the gas.
The Combined Gas Law
What happens if none of the variables for a gas are constant (pressure, volume, temperature, and moles of the gas were changed)? The result would be the Combined Gas Law. Let's derive this law. Give pressure, volume, moles and temperature subscripts, since they are all changing:
Collect terms. Divide each equation by their respective mole and temperature term:
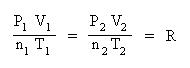
This equation is very useful since it contains any empirical gas law relationship you may need to come up with. If moles and temperature are held constant, then the above equation simplifies down to Boyle's Law. If pressure and moles are held constant, then the equation simplifies down to Charles' Law, etc. If only moles are held constant, then substitute the known pressure, volume and temperatures into the above equation and solve for the unknown quantity. For instance, suppose you had a gas at 15.0 atm pressure, at a volume of 25.0 L and a temperature of 300 K. What would the volume of the gas be at standard temperature and pressure? Standard pressure is 1.00 atm and standard temperature is 0 oC (or 273 K)
.
Set up a table of knowns and unknown, as before:

Substitute these variables into the combined gas law. Since the moles are unchanged, the mole terms have dropped out of the equation:
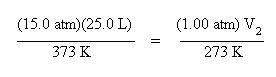
Solve the volume, V2. Multiply both sides of the equation by 273 K and divide both sides of the equation by 1.00 atm:
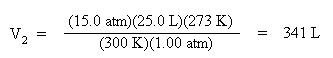
Other Equations Derived from the Ideal Gas Law
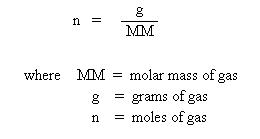
Substitute this into the ideal gas law, and one obtains the equation:
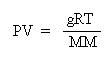
Multiplying both sides by the molar mass, MM, obtains:
This equation is useful for determining the molar mass of a gas from experimental data, where the mass, pressure, volume and temperature of the gas is measured.
Now, let's divide both sides of the above expression by the volume, V:
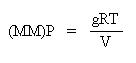
Since we know that g/V is density, D, let's substitute density in for g/V in the above equation:
This equation is useful for relating the pressure, density and temperature of a gas, in the same way as the other empirical gas laws we have encountered.
Real Gases
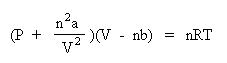
Notice how "corrections" are being made to the pressure term and the volume term. Since collisions of real Gases are non-elastic, the term n2a/V2 is correcting for the interactions of these particles. The value of a is a constant, and must be experimentally determined for each gas. Since real gas particles have real volume, the nb term is correcting for the excluded volume. The value of b is a constant, and must be determined experimentally for each gas. The van der Waals constants, a and b for many gases have been tabulated in the CRC Handbook of Chemistry and Physics. Needless to say, they would be given to you if you are required to solve a problem using this equation. At this level, only pressure or temperature can be solved for easily. Solving for the volume is nontrivial and involves solving a cubic polynomial equation. Let's show how the van der Waals equation is rearranged to solve for pressure. Begin by dividing both sides of the equation by the volume term, V - nb:
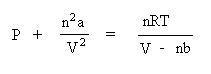
Next, subtract the interparticle interaction term, n2a/V2 from both sides of the equation:
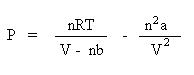
Using the real gas law, let's find the pressure of 2.00 moles of carbon dioxide gas at 298 K in a
5.00 L container. The van der Waals constants for carbon dioxide are: a = 3.592 L2. atm/mol2 and b = 0.04267 L/mol.
Substituting all of the variables into the appropriate terms of the equation, one obtains the pressure of :

Compare this to the pressure calculated using the ideal gas law: