When you write a mechanism, you do not have to include the reaction (energy) diagram, just the steps showing all the intermediates. Here are the conventions for writing a particular mechanism:
- Show all intermediates that you know about as separate sequential drawings (part E gives tips for figuring out what might come next).
- Link all intermediates by straight arrows, double if you know the step is reversible and
single if you know it is not. Each set of arrows followed by a new structure is a step.
- Show one change in bonding for each step (e.g. for E1: ionization, removal of proton), unless you know that more than one bond is changed in a given step (e.g. E2).
- If there are steps that you have little evidence about because they are after the rate determining
step, use analogies to other known reactions to fill in the blanks (e.g. loss of a proton after an
acid-catalyzed reaction)
- If necessary, add an intermediate to the set you know about, again using analogies to other known reactions, to ensure that only one bond-making / bond-breaking occurs for each step.
- If there are no known intermediates, sketch the transition state and label it as such (see F).
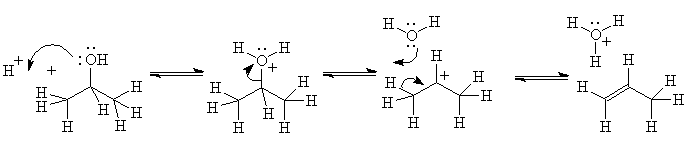
Here is an annotated example using the dehydration of an alcohol:
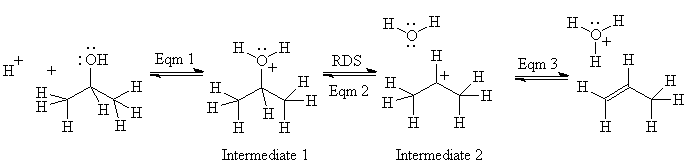
Equilibrium 1: reaction is acid-catalyzed; spectroscopy shows the conjugate acid of the alcohol, intermediate 1, is formed very fast - proton transfers are almost never rate-determining steps for other reactions.
Equilibrium 2: the rate determining step (acid and alcohol concentrations affect the rate). Evidence for a carbocation, intermediate 2? With all alcohols, some substitution is observed, more if the acid is something like HBr, whose conjugate base is nucleophilic; with some alcohols, rearrangement occurs. Both of these observations are consistent with carbocation formation (and not with concerted, carbanion or radical reactions)
Equilibrium 3: This reaction cannot be readily observed under these reaction conditions since it is after the rate-determining step. However, we observe separately that alkenes dissolve
in concentrated sulfuric acid, and thus must undergo an acid-base reaction themselves (protonation) to form soluble ions, which must be carbocations.
Note that this whole reaction is reversible, and in fact, alkenes can be hydrated to form alcohols. How would you change the conditions to produce alcohol as the major product from this equilibrium?
E. Understanding and Predicting Mechanisms
To help us understand how and why these steps occur, we add one important detail to the outline of a
mechanism above: we show how the electrons are used. For the bonds to break and form,
electrons must change their affiliation: unshared become shared, shared with one atom become
shared with another.
We illustrate this dynamic process with a curved arrow for each electron pair which
- starts in the middle of the original location of the electron pair,
- ends at the middle of the final location of the electron pair, as shown below, and
- uses the electrons at a negative or d- site for binding to positive or d+ site.
To avoid confusion, arrows may never be used to show the motion of molecules or ions.
Note that this convention for drawing mechanisms is a shorthand. What is "really" happening is
that atoms are rehybridizing and otherwise reorganizing orbitals to adjust to new bonding
patterns. The arrows show what electron reorganization has to occur to convert the structure with the arrows into the next one in the sequence of steps in the mechanism, i.e. the structure after the arrow. Our shorthand does not automatically show stereochemistry - we have to arrange the
molecule so that we convey that information too.
These arrows are powerful tools to help clarify our thinking about mechanism. They give us a formalism to show how bonds are broken and made during a reaction which allows us to predict reactions that might occur in new compounds with new reagents. They are very useful for keeping track of what does happen - if you use the arrows, they will help you remember the mechanism without memorizing a sequence of structures. Some instructors require that they be included in the mechanism that you write. Learn to use them and it will make your life easier.
The curved arrow notation is also very good at showing the effect of resonance stabilization on a
reaction - the arrow notation is also used to illustrate the relationship between contributors to a
resonance hybrid. If your drawings include contributors to a resonance hybrid, enclose all the
sketches of the same molecule in square brackets (the standard connection is a double-headed
arrow, but you can omit that) to let people know that the sequence of structures is a set of
drawings of one molecule. See the tips by Liina Ladon for further help.
F. Mechanisms without Intermediates
If experiments indicate that no intermediates exist, that the reagents are converted to products in one step, the reaction is said to be "concerted". Such reactions are even called "no mechanism" reactions. Many of them are stereospecific (e.g. E2 and SN2), and we know from the rate law what ingredients go into the transition state, so we do know a lot about how they happen. We do in fact know the mechanism - it is just short. To tell people what we know, we try to make a sketch of the transition state. There are two ways to do this: with curved arrows or with dotted lines (the dotted lines are a simplified version of a molecular orbital picture). The E2 reaction is shown below in both notations. Be sure your transition state is in parentheses to indicate its instability and labeled as such. The character traditionally used for transition state does not exist for html, so I have tried to generate it with the drawing program.
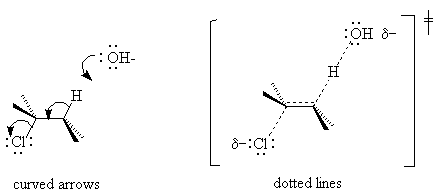
For more examples of concerted and step-wise reactions, see the essay by Drs. Ryzhkov and Wingrove on the SN1, SN2, E1 and E2 reactions.
© Linda M. Sweeting, December 1998. Last revised December 1998.