- To make monocarbonyl compounds, use the aldol condensation.
Note: crossed or mixed aldol condensations (2 different aldehydes or ketones), work much better if one of the reagents has no alpha-hydrogens.
- beta-hydroxyaldehydes and ketones
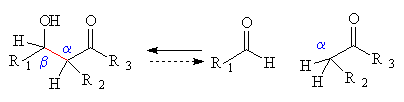
The product can also be transformed by:
- elimination (see b),
- addition to the carbonyl by all the normal reagents, e.g. Grignard, LiAlH4, alchols, amines.
- alpha,beta - unsaturated (conjugated) aldehydes and ketones

The product can also be transformed by:- reduction to a saturated alcohol by H2, Pt, heat and pressure;
- addition by a nucleophile (Nuc-) to the unsaturated carbonyl compound either at the carbonyl carbon or the beta-carbon, both of which are somewhat positive.

The common nucleophilic reagents react as follows:beta only, mostly reversible reactions either C=O or beta, depending on steric factors, irreversible carbonyl only, irreversible reactions OH-, CH3O- RMgX LiAlH4 NH2R, NC- RLi Wittig, Ph3P=CHR enolates
R2CuLi (irrev)
- elimination (see b),
- beta-hydroxyaldehydes and ketones
- To make beta-dicarbonyl compounds, use the Claisen condensation.
Notes:
- crossed or mixed Claisen condensations (2 different esters), work much better if one of the reagents has no alpha-hydrogens.
- the base catalyst must be the conjugate base of the alcohol used tomake the ester, to prevent hydrolysis or exchange
- the product does not react further because it is immediately converted to its conjugate base, the weakest base in the reaction mixture.
- esters, aldehydes and ketones may be used together in condensation reactions, and either one may be the source of the enolate.
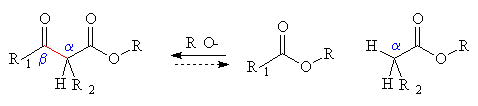
The product can also be transformed by:- reduction (it is possible to reduce both carbonyls or to selectively reduce either the ester or the ketone)
- hydrolysis and decarboxylation
- SN2 reactions of its enolate.
- To make delta-dicarbonyl compounds, use the Michael condensation
Notes:
- be sure only one enolate can form (or one is formed very preferentially); a good way to do this is by using an R2 which begins with another carbonyl
- be sure your base catalyst is consumed or not nucleophilic (not the reactive ones listed in the table in 1b)
- esters, aldehydes and ketones undergo this reaction in either role

The product can also be transformed by:- reduction of the carbonyls
- other reactions at the carbonyls or alpha carbons, e.g. further condensations such as the Robinson annulation