
Internal Energy
This equation is often applied to the first law of thermodynamics. This law states that the energy of the universe remains constant, or energy can be neither created nor destroyed, only changed from one form of energy to another.
The sign of q, heat, or work, w, indicates the direction of the flow of energy. The currently accepted sign convention is that if heat flows out the system to the surroundings, q is negative. If one were carrying out a reaction in a test tube, the test tube would feel warmer. If heat flows into the system from the surroundings, q is positive. If one were carrying out the reaction in a test tube, the test tube would feel colder. If the system does work on the surroundings, w is negative. This means that energy is flowing out of the system. If the surroundings do work on the system, w is positive. Energy is flowing into the system from the surroundings. The way to keep the signs straight is to relate them to what is happening to the system. Heat or energy flowing out of the system is negative; heat or energy flowing into the system is positive. Unfortunately, some areas of science choose to follow the opposite sign convention. It is important to note in a text which sign convention is used.
Note the use of appropriate signs for heat and work. Overall, energy has flowed out of the system to the surroundings by -8.2 kJ.
The change in volume, ![]() V is always calculated as the final volume of the gas less the initial volume of the gas:
V is always calculated as the final volume of the gas less the initial volume of the gas:
To expand a gas, the volume of the gas is increased (![]() V is positive). The gas (part of the system) has to do work on the surroundings, and thus the work must be negative. Similarly, to compress a gas, the volume of the gas is decreased
V is positive). The gas (part of the system) has to do work on the surroundings, and thus the work must be negative. Similarly, to compress a gas, the volume of the gas is decreased ![]() V is negative). The surroundings must do work on the system, and thus the work must be positive.
V is negative). The surroundings must do work on the system, and thus the work must be positive.
Enthalpy
Heat of Formation
where n represents the moles of each reactant or product as found in the balanced chemical equation. The Greek letter,![]() , means one takes the sum of the variables which follow. When choosing
, means one takes the sum of the variables which follow. When choosing ![]() Hfo values from a table, make sure you choose the value corresponding to the appropriate state of matter (solid, liquid, aqueous, gas). Also, note that the standard heat of formation for an element in its standard state always has a value of 0 kJ/mol.
Hfo values from a table, make sure you choose the value corresponding to the appropriate state of matter (solid, liquid, aqueous, gas). Also, note that the standard heat of formation for an element in its standard state always has a value of 0 kJ/mol.
From a table of thermodynamic quantities, one can gather the appropriate values for the heats of formation of each the components in the reaction, and set up the equation to calculate the heat of reaction:
Since ![]() Hrxno is negative, the reaction is an exothermic process. Heat is released by the system to the surroundings. This particular reaction commonly occurs in the vents of volcanos, and deposits sulfur at the entrance of the vents.
Hrxno is negative, the reaction is an exothermic process. Heat is released by the system to the surroundings. This particular reaction commonly occurs in the vents of volcanos, and deposits sulfur at the entrance of the vents.
Hess' Law
from the following heats of reaction:
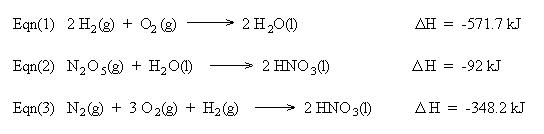
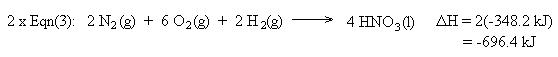
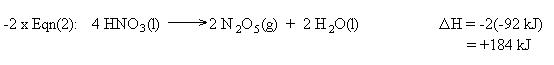
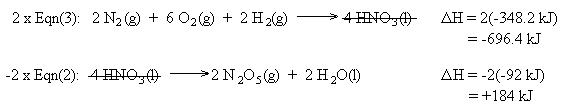
We also need to cancel two moles of H2 , two moles of H2O, and one mole of O2. This is accomplished using equation (1) in reverse. Remember to reverse the sign for the heat of reaction:
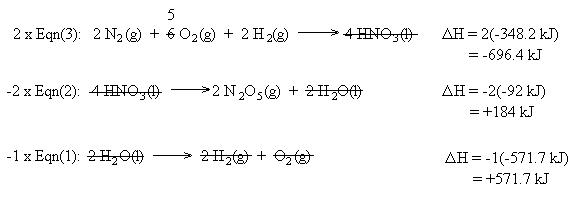
Summing the chemical equations gives us the sought reaction. Summing the heats of reaction gives us the heat of reaction for the overall process:
Calorimetry

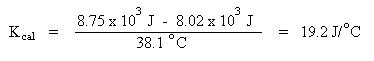
Once again set up the problem based on the heat flow:
Combining terms gives:
Solving for specific heat capacity:
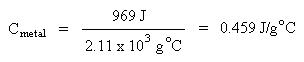
Entropy
Entropy is a measure of the randomness or disorder in a system. The second law of thermodynamics states that for a spontaneous process, the entropy of the Universe will increase. Implicit in this statement is that to create order takes energy. Let's look at an example describing the second law of thermodynamics. To mimic a spontaneous process, suppose you had a sudden urge to take a piece of paper and tear it up into small pieces, and throw the pieces into the air. What would happen? The pieces of paper will randomly scatter about you. They certainly would not fly back together into the original piece of paper. You have just increased the entropy of the Universe. It did not take much energy to tear of the piece of paper and toss the pieces in the air. Now suppose you want to restore this piece of paper into its original form. How much energy would that take? First you have to expend some energy to go around the room and pick up the pieces of paper. The paper is still not restored to its original state. Next, you have to take the pieces of paper to a pulp mill and have the paper turned back into pulp and repressed into the original piece. These processes certainly took much more energy than the energy required to rip up the paper and toss it in the air.
where n represents the moles of each reactant or product, as given in the balanced chemical equation.
Collect terms, and make sure that the mathematical signs are accounted for:
Solving for the entropy of reaction gives:
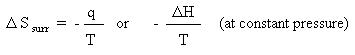
Using the above equation:
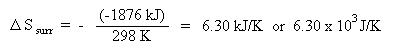
Overall, the entropy has increased. According to the second law of thermodynamics, this implies that the reaction we have been discussing is spontaneous. The best way to determine the spontaneity of a reaction is by looking at the next topic, Gibb's Free Energy.
Gibb's Free Energy
As mentioned, Gibb's free energy, ![]() G, determines whether a reaction is spontaneous or nonspontaneous. If
G, determines whether a reaction is spontaneous or nonspontaneous. If ![]() G < 0, the reaction is spontaneous; if
G < 0, the reaction is spontaneous; if ![]() G > 0, the reaction is nonspontaneous; if
G > 0, the reaction is nonspontaneous; if ![]() G = 0, the reaction is at equilibrium. There are numerous ways to calculate Gibb's free energy. One of the methods will be analogous to previous methods discussed for enthalpy and entropy. The equation is shown below:
G = 0, the reaction is at equilibrium. There are numerous ways to calculate Gibb's free energy. One of the methods will be analogous to previous methods discussed for enthalpy and entropy. The equation is shown below:
where n represents the moles of each product or reactant is given by the coefficient in the balanced chemical equation. As with enthalpy, you will find that the standard Gibb's free energy of formation of elements in their standard states is 0 kJ/mol.
Set up the equation:
From tabulated data, substitute for the Gibb's free energies:
Combine terms, paying careful attention to the mathematical signs:
As predicted, the reaction is spontaneous, since ![]() Gorxn <0.
Gorxn <0.
From previous calculations, we have determined that the enthalpy change was -1876 kJ, the entropy change was -4.065 kJ/K, and the temperature has a value of 298 K (standard temperature). Notice that is important to make sure the units are consistent, so entropy, which is often expressed in units of J/K has been converted to kJ/K. Substituting these quantities into the above equation gives:
This answer agrees reasonably well with the result from the calculation from the Gibb's free energies of formation.
where R is the ideal gas law constant, 8.314 J/mol-K, T is temperature in kelvin, and K is the equilibrium constant for the chemical equilibrium taking place. The second equation is:
where n represents the moles of electrons transfered in an electrochemical process, F is Faraday's constant, 96,485 C/mol, and Eo is the standard potential for an electrochemical cell.