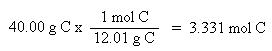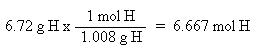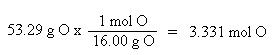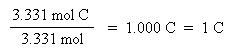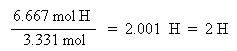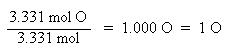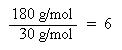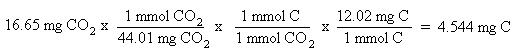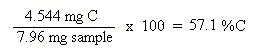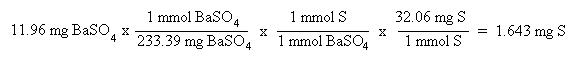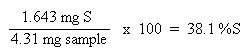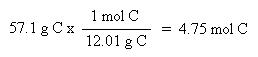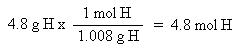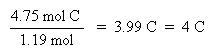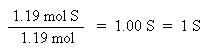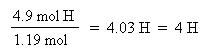Now that the moles of each element are known, the empirical formula may be determined by dividing the moles of each element by the smallest number of moles. This yields a ratio of the number of each element in the empirical formula.
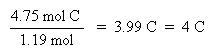
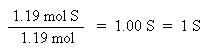
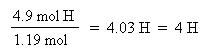
The ratio of C:H:S has been found to be 4:4:1, thus the empirical formula is: C4H4S. The molar mass of the empirical formula is 84 g/mol. Since the molecular weight of the actual compound is 168 g/mol, and is double the molar mass of the empirical formula, the molecular formula must be twice the empirical formula:
C(4 x 2) H(4 x 2) S(1 x 2) which becomes C8H8S2
Created With HTML Assistant Pro - 05/11/2001
© Copyrght, 2001, L. Ladon. Permission is granted to use and duplicate these materials for non-profit educational use, under the following conditions: No changes or modifications will be made without written permission from the author. Copyright registration marks and author acknowledgement must be retained intact.