
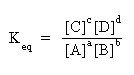
Qualitative Problems
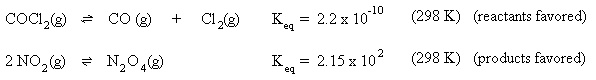
Suppose an inert gas, such as helium, as added to the system. Since helium is not a part of this equilibrium, it will not affect the equilibrium.
Since the process is exothermic, heat is released by the system to the surroundings, so one may include heat in the equilibrium as a product:
Suppose the temperature of the system is increased to 500 K. The stress is too much heat, so the equilibrium will shift to the left to remove the excess heat. In addition, the value of the equilibrium constant decreases to 4 x 1018 , indicating the equilibrium lies further to the left.
Notice that the solids were not included as part of the mass action expression.
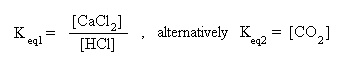
Notice that the equilibrium mass action expression may be expressed in two ways; Keq1 is expressed in terms of the concentrations of the aqueous species, while Keq2 is expressed in terms of the concentration of the gaseous species. These two constants will have different values. Which expression do you use? That depends on the information given in the problem. If the concentrations of the aqueous species are given, use the expression for Keq1 . If the concentration for CO2 is given, use the expression for Keq2 . Realistically, one uses the expression whose quantities are most easily measured experimentally. The main idea is not to mix phases, and that the solid is not included in either expression.
Quantitative Problems
The equilibrium concentrations are: [CO] = 0.0911 M, [H2] = 0.0822 M, [CH3OH] = 0.00892 M, what is the value of the equilibrium constant? Does the equilibrium favor reactants or products?
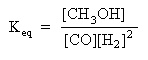
Next, substitute the equilibrium concentrations into the mass action expression, and calculate for the equilibrium constant:
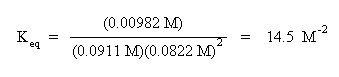
If the initial amounts of CO and H2O were both 0.100 M, what will be the amounts of each reactant and product at equilibrium?
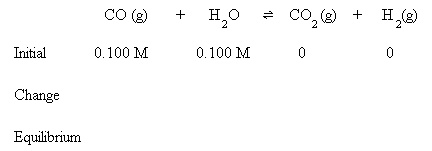
Initially, 0.100 M CO and 0.100 M H2O are present. Equilibrium hasn't been established yet, so the amounts of CO2 and H2 are assumed to be zero.
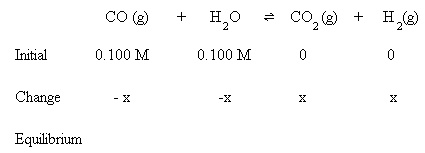
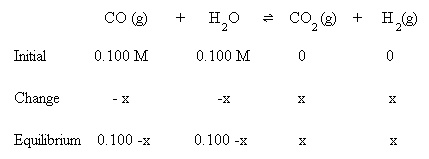
Substitute the above algebraic quantities into the mass action expression:
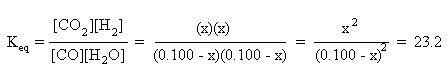
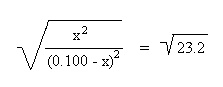
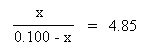
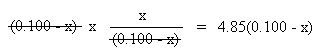
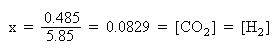
© Copyright, 2001, L. Ladon. Permission is granted to use and duplicate these materials for non-profit educational use, under the following conditions: No changes or modifications will be made without written permission from the author. Copyright registration marks and author acknowledgement must be retained intact.