Let's look at an example of each of the hybridizations of carbon.
For our first example, let's choose methane, CH4 . Draw the Lewis structure:
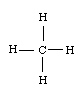
The Lewis structure shows four groups around the carbon atom. This means four hybrid orbitals have formed. In order to form four hybrid orbitals, four atomic orbitals have been mixed. The s orbital and all three p orbitals have been mixed, thus the hybridization is sp3 .
Let's show this using the atomic orbitals of excited state carbon found in the valence shell:

The four sp3 hybrid orbitals will arrange themselves in three dimensional space to get as far apart as possible (to minimize repulsion). The geometry that achieves this is tetrahedral geometry, where any bond angle is 109.5o.
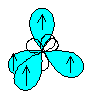
Each hybrid orbital contains 1 electron. A hydrogen 1s orbital will come in and overlap with the hybrid orbital to form a sigma bond (head-on overlap), as shown below:
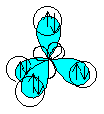 means the same as
means the same as 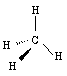
Next, let's choose ethene, C2H4 . Draw the Lewis structure:
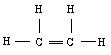
The Lewis structure show three groups around each carbon atom. This means three hybrid orbitals have formed for each carbon. In order to form three hybrid orbitals, three atomic orbitals have been mixed. The s orbital and two of the p orbitals for each carbon have been mixed, thus the hybridization for each carbon is sp2 .
Let's show this using the atomic orbitals of excited state carbon found in the valence shell:
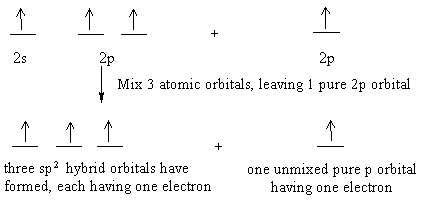
The three sp2 hybrid orbitals will arrange themselves in three dimensional space to get as far apart as possible. The geometry that achieves this is trigonal planar geometry, where the bond angle between the hybrid orbitals is 120o. The unmixed pure p orbital will be perpendicular to this plane. Keep in mind, each carbon atom is sp2 , and trigonal planar.
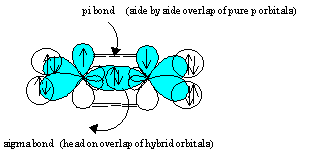
Notice that the head-on overlap of sp2 orbitals forms a bond and the side by side overlap of pure p orbitals forms a pi bond between the carbon atoms. This accounts for the carbon-carbon double bond.
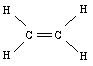
Each carbon is trigonal planar with a bond angle of 120o.
Finally, let's look at acetylene, C2H2 . First, draw the Lewis structure:

The Lewis structure shows two groups around each carbon atom. This means two hybrid orbitals have formed. In order to form two hybrid orbitals, two atomic orbitals have been mixed.
Let's show this using the atomic orbitals of excited state carbon found in the valence shell:
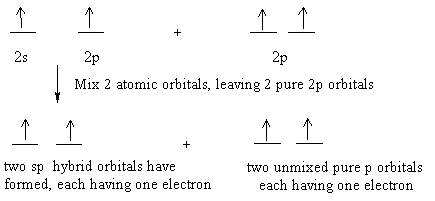
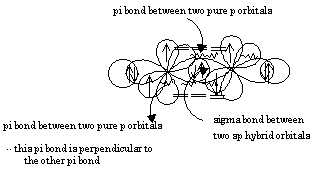
The two sp hybrid orbitals arrange themselves in three dimensional space to get as far apart as possible. The geometry which achieves is linear geometry with a bond angle of 180o. The two pure p orbitals which were not mixed are perpendicular to each other.
 same as
same as 
The triple bond consists of one sigma bond and two pi bonds. The geometry around each carbon is linear with a bond angle of 180o.
Created With HTML Assistant Pro - 05/11/2001
© Copyrght, 2001, L. Ladon. Permission is granted to use and duplicate these materials for non-profit educational use, under the following conditions: No changes or modifications will be made without written permission from the author. Copyright registration marks and author acknowledgement must be retained intact.
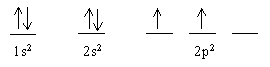
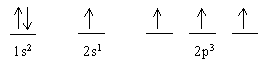


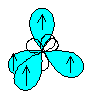
 means the same as
means the same as 
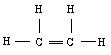


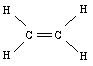

 same as
same as