
SCIENTIFIC EXPERIMENTS AT HOME:
WINTERGREEN CANDY AND OTHER TRIBOLUMINESCENT MATERIALS
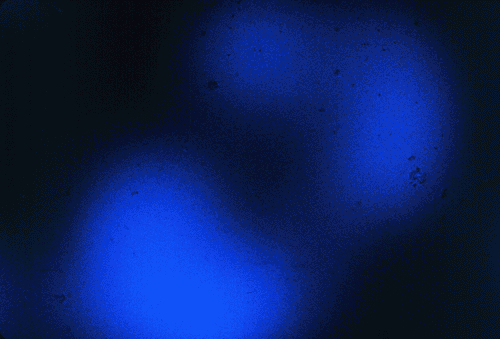
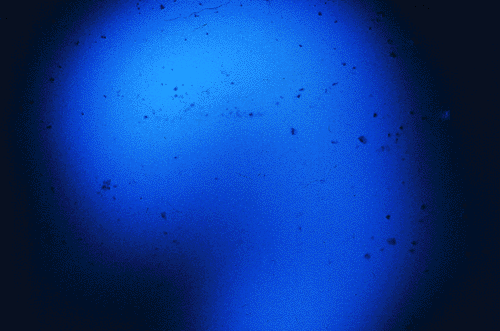
Figure 1. Photograph of the emission of WintOGreen Lifesavers, taken by Towson State University undergraduate students. Kodak Ektachrome (EL) color film (ISO 400) was placed under a sheet of Plexiglas; the candy was placed on top of the Plexiglas, covered with another piece of Plexiglas and smashed with a hammer.
Note: this little essay has some questions in it. If you see a link, the question is answered if you follow the link. If you don't, the question is answered in the next few sentences.
Find yourself a room with no windows (a closet or bathroom will do). Take with you sugar cubes, wintergreen (and other) candies, adhesive tape (any kind will do) and a mirror or friend. It would be helpful to partially open the candy and make a pull-tab on the end of the tape roll. Go into the closet and wait 5-10 minutes. During that period, your eyes will adapt to the darkness and you will begin to be able to see a little of your surroundings (unless you are in a real "darkroom"). Take two sugar cubes and strike one against the other as if you were trying to strike a match. If your eyes are sufficiently adjusted, you will see a bluish white glow from the sugar cubes when you strike them. (Some people find the glow greenish - our ability to detect color when our eyes are dark-adapted is not very good.) Now for the candy. Place the candy between your teeth, keeping your mouth open, and bite down. Your friend will see blue light coming from your mouth like that shown above in Figure 1 (or you can see it yourself using the mirror). Once the candy gets soggy, it will not produce any light, so work quickly. We have found that this works on all opaque candy made with sugar: transparent candy doesn't work, nor does saccharin- or aspartame-based candy. The tape experiment is easy. Just grab that pull-tab and pull it some tape off the roll. You can stick it back down and repeat the experiment or waste a lot of tape, as you wish.
You can make these experiments a little fancier, if you like. We built a Plexiglas holder, unrolled the film onto it (in the darkroom, of course), covered it with more Plexiglas and put WintOGreen Lifesavers (TM) on top. When we smashed them with a hammer, we got images like the one in Figure 1. We had no luck taking pictures with a camera; we think the camera aperture collects too small a fraction of the emitted light, especially compared to our method. Dickinson and Donaldson (professors at Washington State University) have worked a lot on adhesives; they applied special powders after tearing adhesive tape off a surface and found there were residual patches of positive and negative electrical charge on the tape; these experiments are described in a Scientific American article.
This fascinating phenomenon has been known for centuries and has several names: triboluminescence (from the Greek tribein, to rub), mechanoluminescence and fractoluminescence (a part of fracto-emission) - luminescence (light emission) from rubbing, mechanical action and fracture. I've been studying this phenomenon for more than 10 years, with help from undergraduate students and other professional scientists and will tell you what we have learned. Be forewarned, this is more of an essay than a flashy web site, and we'll start at the beginning so almost everyone can understand with notes to explain some things that you may not have learned in school (accessible by links).
Luminescence usually occurs if you supply a lot of energy to atoms, for example by heating them in a flame or by passing electricity through them; they emit light of a color that is characteristic of the kind of atom. If the spectrum of the light is examined to determine the colors that comprise the light, it is found to consist of sharp lines, i.e. very specific colors. Niels Bohr explained these sharp lines by proposing that electrons in atoms have only certain allowed energies, just as you are only allowed to have only certain energies and positions when you are standing on stairs - you can't stand between the steps. When the electrons are transported up the energy stairs (excited) by the heat or electricity, they are also able to go back to the lowest energy (the ground floor) by steps. As they cascade down the steps, the energy they lose is released as light whose color is determined by the size of the step (there are many different sizes of steps in each atom). Molecules behave the same way except that, because their atoms move relative to each other, the steps are sloped and uneven. Individual electrons will land on different parts of the rough steps and thus the energies are a little different with each event, but the emission lines so close together we cannot distinguish them. Figure 2 below shows the spikes from the emission of a simple molecule - nitrogen gas; the emission spectra of atoms are even sharper than these. A complex molecule like wintergreen (Figure 3) has so many overlapping, rough steps that emission lines are all on top of each other and the overall emission is very broad. For more information about the meaning of the graphs, see the appendix.
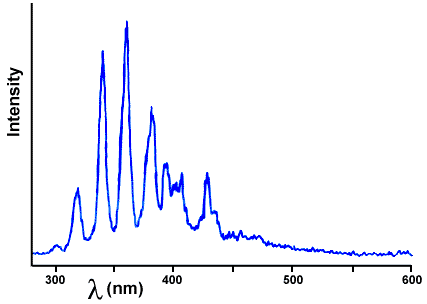
Figure 2. Triboluminescence spectrum of sucrose. The observed lines are the emission of nitrogen when electricity discharges through it, as it does during a lightning bolt. We are grateful to EG&G PAR of Princeton, NJ for letting us use their equipment.
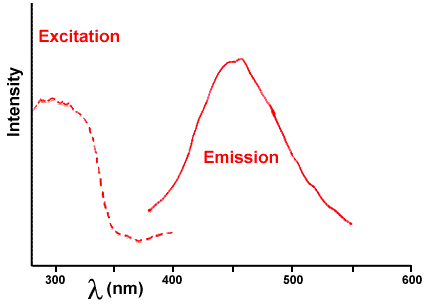
Figure 3. Fluorescence spectrum of wintergreen (methyl salicylate). The solid line is the emission spectrum and the dotted line is the energy it absorbs to produce that emission.
Our eyes are able to see only a small fraction of the light that atoms and molecules emit, namely that between violet (big steps) and red (smaller steps) which we call "visible". Light we cannot see is called ultraviolet (the high energy emissions which cause sunburn) and infrared (which we can detect as heat). The spectra above contain both visible (>380 nanometers, nm) and ultraviolet emissions (<380 nm), but no infrared (< 780 nm).
My research at Towson U has been focussed on how the light is produced during triboluminescence, and in particular, what causes the electrons to be excited. The mechanical energy you put into materials to break them is only enough to move the electrons around on the steps, and not enough to push them up to another step. Following in the footsteps of a French scientist in the 1930's, we have examined the spectrum of the light emitted and thus the arrangement of the electrons falling down the energy steps.
The spectrum of the light produced during the triboluminescence of sugar partially solves the mystery - it is exactly the same as the spectrum of lightning! This spectrum is shown above in Figure 2. Where does lightning come from? From an electrical current (electrons) passing through air, exciting the nitrogen molecules of the air. It appears that triboluminescence is lightning on a very small scale. When the sugar is cracked, electrical charge is separated, positive from negative, and when there is a big enough charge accumulation (electric field) the electrons jump through the air in the crack, colliding with and exciting the nitrogen molecules as they do. Most of the light emitted by the nitrogen in the air is ultraviolet (wavelengths less than 380 nm in Figure 2), at too high an energy for our eyes to detect it; a small fraction is in the visible region and thus the emission appears pretty faint.
The emission from wintergreen candy is much brighter - why is that so? The triboluminescence spectrum of WintOGreen Lifesavers (TM) in Figure 4 tells us. The emission has a broad band from 400 to 500 nm in addition to the lightning lines produced by the sugar. Thus much more of the candy emission than the sugar emission is in the region of the spectrum our eyes can detect (from about 380 - 780 nm). So it may not be brighter after all; the apparent brightness is probably caused by the fact that more of the light is visible (rather than ultraviolet). But what is the broad visible light caused by? It certainly isn't lightning!
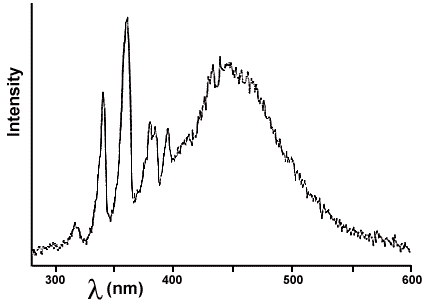 Figure 4. Triboluminescence emission spectrum of WintOGreen Lifesavers.
Figure 4. Triboluminescence emission spectrum of WintOGreen Lifesavers.
Certain flavorings, such as wintergreen, are themselves luminescent (fluorescent or phosphorescent). For them to emit light, they must absorb light of higher energy (shorter wavelength). If you examine the fluorescence of oil of wintergreen by shining ultraviolet light on it, it absorbs ultraviolet light (dotted line in Figure 3) to excite its electrons up those rough steps and then emits visible light (solid line in Figure 3) as they fall down. That emission (solid line) is an excellent match to the broad band in the candy triboluminescence spectrum of Figure 4, so the wintergreen fluorescence is probably the source of that emission. The two spectra are shown together in Figure 5.
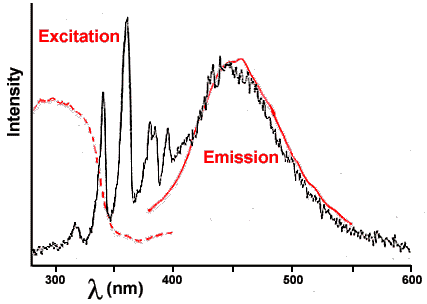 Figure 5. Comparison of the triboluminescence emission of WintOGreen Lifesavers from Figure 4 with the fluorescence of oil of wintergreen from Figure 3.
Figure 5. Comparison of the triboluminescence emission of WintOGreen Lifesavers from Figure 4 with the fluorescence of oil of wintergreen from Figure 3.
And by a wonderful coincidence, the light that wintergreen likes to absorb (dotted line) is a good match to the lightning emissions produced by the sugar; they are shown together in Figure 6.
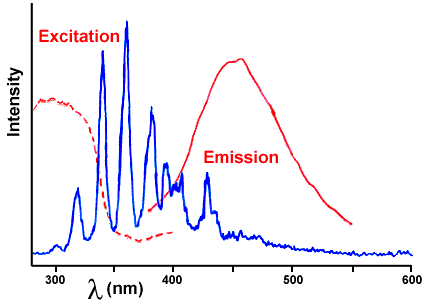 Figure 6. Comparison of the emission of sugar (nitrogen emission from lightning) from Figure 2 with the fluorescence of oil of wintergreen from Figure 3.
Figure 6. Comparison of the emission of sugar (nitrogen emission from lightning) from Figure 2 with the fluorescence of oil of wintergreen from Figure 3.
We believe that the ultraviolet lightning emission from sugar is responsible for exciting the wintergreen: if you look carefully at the triboluminescence emission of WintOGreen Lifesavers, you can see that some of the lightning emission lines have been absorbed more than others and are depleted in the spectrum. Figure 7 compares the lightning triboluminescence emission from pure sugar to the triboluminescence of the Lifesavers. The most intense lightning line from sugar is at 338 nm, but in the emission from the Lifesavers the 338 nm line is smaller than that at 357 nm; we have had to shift the sugar emission spectrum down to make it possible to see both, but you can still see that the relative intensity of the lightning lines is different in the two spectra.
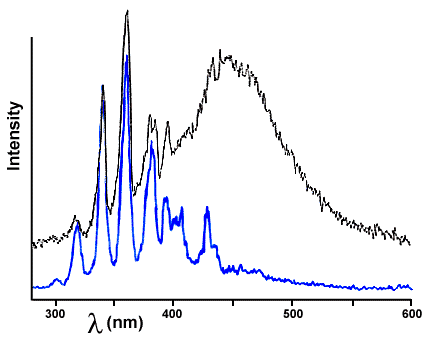 Figure 7. Comparison of the triboluminescence emission of sugar (from Figure 2) with that of WintOGreen Lifesavers (from Figure 4).
Figure 7. Comparison of the triboluminescence emission of sugar (from Figure 2) with that of WintOGreen Lifesavers (from Figure 4).
We are still trying to understand why all materials are not triboluminescent. For example, transparent sugar candy, or candy made with artificial (non-nutritive) sweetener is not. Then again, perhaps our detectors (eyes) are not yet sensitive enough! Triboluminescence seems to be related to piezoelectricity. Piezoelectric materials generate an electrical voltage from separation of positive and negative charges when they are squeezed or stretched; they also change their shape if an electrical voltage is applied. Can you think of a use for such materials? The lightning spectrum and the test for electrical charge with powders on adhesive tape are a good indication that electrical charge is involved in triboluminescence so this looks like a good connection to explore.
A material that is piezoelectric should lack mirror symmetry, that is at least one pair of ends of the crystal must be different from each other. How can a crystal lack symmetry? Imagine making a crystal by arranging a group of cups. If all the cups are upright, the top of the arrangement of cups is different from the bottom. You could also make one side different from the other by aligning all the handles or, even better, putting a logo on one side. You can take the logoless cups and make a symmetrical crystal by alternating upside-down and right-side-up and left-right handles. If all the cups are facing up, then perhaps the open ends can hold electrons better than the closed ends. At the molecular level, such a dissymmetric material has its ability to hold electrons changed when it is squeezed or stretched, and thus electrical charges can be developed or changed - imagine a rubber coffee cup - the volume of coffee it can hold is much smaller if it is squashed. It turns out that these dissymmetric, piezoelectric materials are more likely to be triboluminescent than the symmetric ones. However, about a third of the triboluminescent materials are not piezoelectric and there are some piezoelectric materials that are not triboluminescent. Only a few scientists are working on this problem, but they have found that impurities, disorder and defects are also common in triboluminescent materials (cups mixed with the occasional glass, the occasional one up when it should be down, or a whole row the wrong way). Such errors in the arrangement of the molecules could also allow for electrical charge to collect.
We have also been looking at simple experiments to examine the importance of charge. Remember that the candy emission goes away if the candy gets wet. Electrical discharges are much less likely under liquids, and much less likely in gases with big energy steps like helium. So we have been doing some simple things, such as looking at the emission under liquids and other gases. We hoped to extinguish all the triboluminescence, but we didn't, so we're trying to understand whether the basic idea of a discharge followed by luminescence is applicable to all or just some of the triboluminescent materials.
There is a lot to be done to understand this phenomenon, but when we do we may also learn something about some other important phenomena: earthquake lights (people have seen light from the ground during earthquakes) and the mechanism of explosions! If we understand it well enough we will be able to design materials to be used to sense pressure changes or fracture before they become catastrophic.
A lot of students have used the information on this page in their science fair projects. You may do so as well. It is very important in science to provide references for all your sources of information, so that people will know whether the information is reliable or not, and so everyone will get credit for the work they do. So to be professional, you should list the URL of this site as a source if you use. If you would like to see what one student has done, visit Katie's web site - and don't forget to give her credit if you use some of her ideas.
For further reading:
(1)
Walker, J. Sci. Amer. 1987, 257 (6), 138 - 141 (December issue). This article describes the tape experiments.
(2)
Linda. M. Sweeting, ChemMatters, 1990, 8(3), 10 - 12 (October issue). This article has basically the same information as you read in the web essay.
(3)
If you want to read something more technical, try:
- Sweeting, L. M.; Cashel, M. L.; Dott, M.; Gingerich, J. M.; Guido, J. L.; Pippin, R. F., III; Rosenblatt, M. M.; Rutter, A. W.; Spence, R. A. "Spectroscopy and Mechanism in Triboluminescence", Mol. Cryst. Liq. Cryst. 1992, 211, 389 - 396.
- J. T. Dickinson, L. B. Brix, and L. C. Jensen, "Electron and Positive Ion Emission Accompanying Fracture of Wint-O-Green Lifesavers and Single Crystal Sucrose," J. Phys. Chem. 1984, 88, 1698.
(you should be at least a college junior science major to try to read these)
Appendix 1. Light Emission.
Upon excitation by high energy light, ultraviolet or even X-Ray, atoms and molecules emit light as the fall down from the higher energies they have been excited to. Usually the emission takes place very fast, with a lifetime of nanoseconds or less. With some materials, the fall is hindered (kind of like a lip on the stairs) and it takes a while for the molecules to lose the energy. The fast emission is called fluorescence and the slow emission phosphorescence. Can you find places where these two phenomena are used?
Appendix 2. Measuring Light
There are two important things we need to know about every kind of light: its color and its intensity or brightness. In the spectra displayed here both color and brightness are shown - and not in the color of the drawings. The color of the light is described by the wavelength of the light; here the wavelength is shown on the horizontal axis using an appropriate unit, the nanometer, abbreviated nm. A meter is a little more than a yard (39.3"); a nanometer is much smaller - 1 nm = 0.000000001 meters. For those of you familiar with powers, 1 nm = 10-9 m. Very small! The waves you see in the ocean have wavelengths of a few meters. The color correspondence is such that we call the shortest wavelength we can see violet, then blue, then green, yellow, orange and red. The color can also be described by energy or frequency: the higher energy and frequency corresponds to the shorter wavelengths - blue has the highest energy and red the least of those we can see. We cannot see infrared light, but we can detect it as heat - did you ever notice that as an electric stove gets hotter and hotter, it eventually glows red? It has become so hot that it is emitting not just infrared but red too.
The intensity of light is usually measured by comparison with a standard. With triboluminescence spectra, comparisons are difficult because of the erratic nature of the emission; we have not shown any values for intensity, the vertical axis, because we only know the relative intensities for each sample. Note - by convention the horizontal axis is nearly always the one considered to be the independent variable, and the vertical axis the dependent variable.
Appendix 3. Symmetry.
Man-made materials have a lot of symmetry because it makes them easier to manufacture. A coffee cup (with no logo) has a plane of symmetry through the handle and across the cup. If you put the coffee cup in front of the mirror, the reflection looks exactly the same. The letter H has the same symmetry in two dimensions as the coffee cup does in three dimensions. Symmetry is often described by telling you what actions or operations to perform to get back the same structure as you started with. Both the coffee cup and the H give the same thing if reflected in a mirror (into the looking glass). A lack of mirror symmetry is called dissymmetry.
If you put your hand in front of the mirror, the image of your right hand is exactly what you would get if you put your left hand behind the mirror and you could see through the mirror. Your right hand reflected gives your left hand, so your hand lacks mirror symmetry. In two dimensions the letter R is a good example of no symmetry at all, or asymmetry.
There is second kind of symmetry, though - the symmetry of a propeller or a fan, which is called rotational symmetry. If you place the fan in front of a mirror, its reflection is a fan that will naturally drive the air the other way. Propellers have more shape, and will only pull an airplane if they turn the correct way; a mirror-image propeller must be rotated the opposite way in order to pull. In two dimensions the letter Z has the symmetry of a propeller; if you rotate 1/2 turn, you get the same thing. Z's mirror image is more like an S; Z and S are not the same, but people sometimes confuse them. If an object gives you the same structure back when you rotate less than a whole turn, then it has rotational symmetry (it must be 1/2, 1/3, 1/4, etc. of a turn).
A third kind of symmetry is hard to describe - and see. Take the letter N, which has rotational symmetry, and put a dot in the exact middle of the letter. Imagine every point on the top being transferred to the bottom by passing them diagonally through that point in the center (and vice versa). This process is called inversion - it's rather like having a mirror that is just a dot, so that each point on one side reflects to each point on the other side.
How would you describe a plain glass of water? Well, you could reflect in any vertical mirror and get the same thing. And you could rotate it the tiniest bit or a whole lot and get the same thing. So it's very symmetrical, but not quite as symmetrical as a solid cylinder. And - you guessed it - the most symmetrical object is a sphere (or a circle in two dimensions).
Appendix 4. Answers to questions.
Piezoelectric materials are used in high-quality speakers and microphones.
Fluorescent materials are used in laundry detergents, so that your clothes emit a bluish-white light under the fluorescent room lights (there's another use - the coating on the inside of the lights). This makes your clothes look bright and white.
Phosphorescent materials are used in computer and TV screens. Electrons hit the screen from behind, excite the electrons and cause the material to phosphoresce. The lifetime is fairly short for these materials, just long enough so that the picture looks continuous while the electron beam is scanning the rest of the screen. Phosphors with longer lifetime are used on watches that glow in the dark.
This site prepared and maintained by Linda M. Sweeting, Professor of Chemistry, Towson University. Last updated September 1998.






 Figure 4. Triboluminescence emission spectrum of WintOGreen Lifesavers.
Figure 4. Triboluminescence emission spectrum of WintOGreen Lifesavers.
 Figure 5. Comparison of the triboluminescence emission of WintOGreen Lifesavers from Figure 4 with the fluorescence of oil of wintergreen from Figure 3.
Figure 5. Comparison of the triboluminescence emission of WintOGreen Lifesavers from Figure 4 with the fluorescence of oil of wintergreen from Figure 3.
 Figure 6. Comparison of the emission of sugar (nitrogen emission from lightning) from Figure 2 with the fluorescence of oil of wintergreen from Figure 3.
Figure 6. Comparison of the emission of sugar (nitrogen emission from lightning) from Figure 2 with the fluorescence of oil of wintergreen from Figure 3.
 Figure 7. Comparison of the triboluminescence emission of sugar (from Figure 2) with that of WintOGreen Lifesavers (from Figure 4).
Figure 7. Comparison of the triboluminescence emission of sugar (from Figure 2) with that of WintOGreen Lifesavers (from Figure 4).